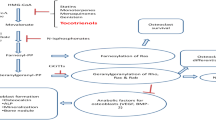
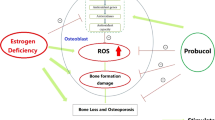
Abstract
Osteoporosis is a major health problem in postmenopausal women globally. This study determined the mechanism through which coelogin stimulates osteoblastogenesis and its osteoprotective and bone regenerating potential. Coelogin effect on primary calvarial osteoblast cells was determined by measuring alkaline phosphatase activity, mineralization, osteoblast survival, and apoptosis and protein expression studies. The osteoprotective effect of coelogin was also evaluated on osteopenic adult female Swiss mice. At autopsy, bones were collected for dynamic and histomorphometry studies. Serum samples were also collected for assessment of serum parameters. Coelogin treatment led to increased osteoblast proliferation, survival, differentiation, and mineralization in osteoblast cells. Coelogin supplementation to Ovx mice promoted new bone formation, prevented Ovx-induced deterioration of bone microarchitecture, and enhanced bone regeneration. In addition, signaling studies revealed that coelogin treatment activates the ER-Erk and Akt-dependent signaling pathways which stimulate the osteoblastogenesis in osteoblast cells.






Similar content being viewed by others
References
Garnero P, Delmas PD (2004) Contribution of bone mineral density and bone turnover markers to the estimation of risk of osteoporotic fracture in postmenopausal women. J Musculoskelet Neuronal Interact 4(1):50
Tabacco G, Tay YD, Cusano NE, Williams J, Omeragic B, Majeed R, Almonte MG, Rubin MR, Bilezikian JP (2019) Quality of life in hypoparathyroidism improves with rhPTH(1–84) throughout 8 years of therapy. J Clin Endocrinol Metab 104:2748–2756. https://doi.org/10.1210/jc.2018-02430
Noel SE, Mangano KM, Griffith JL, Wright NC, Dawson-Hughes B, Tucker KL (2018) Prevalence of osteoporosis and low bone mass among Puerto Rican older adults. J Bone Miner Res 33(3):396–403. https://doi.org/10.1002/jbmr.3315
Reid IR (2008) Anti-resorptive therapies for osteoporosis. Seminars in cell & developmental biology, vol 19. Academic Press, Cambridge, pp 473–478
Deal C (2009) Potential new drug targets for osteoporosis. Nat Clin Pract Rheumatol 5:20–27. https://doi.org/10.1038/ncprheum0977
Anagnostis P, Gkekas NK, Potoupnis M, Kenanidis E, Tsiridis E, Goulis DG (2019) New therapeutic targets for osteoporosis. Maturitas 120:1–6
Wojda SJ, Donahue SW (2018) Parathyroid hormone for bone regeneration. J Orthop Res 36(10):2586–2594
Wein MN, Kronenberg HM (2018) Regulation of bone remodeling by parathyroid hormone. Cold Spring Harb Perspect Med 8(8):a031237
Dobnig H, Turner RT (1995) Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 136:3632–3638. https://doi.org/10.1210/endo.136.8.7628403
Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC (1999) Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 104:439–446. https://doi.org/10.1172/JCI6610
Sen S, Chakraborty R (2017) Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: importance, challenges and future. J Tradit Complement Med 7(2):234–244
Sharma C, Mansoori MN, Dixit M, Shukla P, Kumari T, Bhandari SP, Narender T, Singh D, Arya KR (2014) Ethanolic extract of Coelogyne cristata Lindley (Orchidaceae) and its compound coelogin promote osteoprotective activity in ovariectomized estrogen deficient mice. Phytomedicine 21:1702–1707. https://doi.org/10.1016/j.phymed.2014.08.008
Tripathi JK, Pal S, Awasthi B, Kumar A, Tandon A, Mitra K, Chattopadhyay C, Ghosh JK (2015) Variants of self-assembling peptide, KLD-12 that show both rapid fracture healing and antimicrobial properties. Biomaterials 56:92–103. https://doi.org/10.1016/j.biomaterials.2015.03.046
Pal KK, China SP, Mittal M, Shrivastava R, Taneja I, Hossain Z, Mandalapu D, Gayen JR, Wahajuddin M, Sharma VL (2016) Theophylline, a methylxanthine drug induces osteopenia and alters calciotropic hormones, and prophylactic vitamin D treatment protects against these changes in rats. Toxicol Appl Pharmacol 295:12–25. https://doi.org/10.1016/J.TAAP.2016.02.002
Bhargavan B, Gautam AK, Singh D, Kumar A, Chaurasia S, Tyagi AM, Yadav DK, Mishra JS, SinghAB SS, Goel A (2009) Methoxylated isoflavones, cajanin and isoformononetin, have non-estrogenic bone forming effect via differential mitogen activated protein kinase (MAPK) signaling. J Cell Biochem 108:388–399. https://doi.org/10.1002/jcb.22264
Adhikary S, Choudhary D, Ahmad N, Karvande A, Kumar A, Banala VT, Mishra PR, Trivedi R (2018) Dietary flavonoid kaempferol inhibits glucocorticoid-induced bone loss by promoting osteoblast survival. Nutrition 53:64–76. https://doi.org/10.1016/j.nut.2017.12.003
Meleti Z, Shapiro IM, Adams CS (2000) Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone 27:359–366. https://doi.org/10.1016/S8756-3282(00)00346-X
Jia TL, Wang HZ, Xie LP, Wang XY, Zhang RQ (2003) Daidzein enhances osteoblast growth that may be mediated by increased bone morphogenetic protein (BMP) production. Biochem Pharmacol 65:709–715. https://doi.org/10.1016/S0006-2952(02)01585-X
Nakamura H, Kumei Y, Morita S, Shimokawa H, Ohya K, Shinomiya K (2003) Antagonism between apoptotic (Bax/Bcl-2) and anti-apoptotic (IAP) signals in human osteoblastic cells under vector-averaged gravity condition. Ann N Y Acad Sci 1010:143–147. https://doi.org/10.1196/annals.1299.023
Nagase Y, Iwasawa M, Akiyama T, KadonoY NM, Oshima Y, Yasui T, Matsumoto T, Hirose J, Nakamura H, Miyamoto T (2009) Anti-apoptotic molecule Bcl-2 regulates the differentiation, activation, and survival of both osteoblasts and osteoclasts. J Biol Chem 284:36659–36669. https://doi.org/10.1074/jbc.M109.016915
Calderone L, Grimes P, Shalev M (1986) Acute reversible cataract induced by xylazine and by ketamine-xylazine anesthesia in rats and mice. Exp Eye Res 42:331–337. https://doi.org/10.1016/0014-4835(86)90026-6
Hara K, Kobayashi M, Akiyama Y (2002) Vitamin K2 (menatetrenone) inhibits bone loss induced by prednisolone partly through enhancement of bone formation in rats. Bone 31:575–581. https://doi.org/10.1016/S8756-3282(02)00874-8
Chen HL, Demiralp B, Schneider A, Koh AJ, Silve C, Wang CY, McCauley LK (2002) Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. J Biol Chem 277:19374–19381. https://doi.org/10.1074/jbc.m108913200
Chau JF, Leong WF, Li B (2009) Signaling pathways governing osteoblast proliferation, differentiation and function. Histol Histopathol 24:1593–1606. https://doi.org/10.14670/hh-24.1593
Yang XS, Liu S, Liu YJ, Liu JW, Liu TJ, Wang XQ, Yan Q (2010) Overexpression of fucosyltransferase IV promotes A431 cell proliferation through activating MAPK and PI3K/Akt signaling pathways. J Cell Physiol 225:612–619. https://doi.org/10.1002/jcp.22250
He YX, Zhang G, Pan XH, Liu Z, Zheng LZ, Chan CW, Lee KM, Cao YP, Li G, Wei L, Hung LK (2011) Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: a drill-hole defect model. Bone 48:1388–1400. https://doi.org/10.1016/j.bone.2011.03.720
Garnero P, Sornay-Rendu E, Munoz F, Borel O, Chapurlat RD (2013) Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int 24:489–494. https://doi.org/10.1007/s00198-012-1978-x
He YX, Liu Z, Pan XH, Tang T, Guo BS, Zheng LZ, Xie XH, Wang XL, Lee KM, Li G, Cao YP (2012) Deletion of estrogen receptor beta accelerates early stage of bone healing in a mouse osteotomy model. Osteoporos Int 23:377–389. https://doi.org/10.1007/s00198-011-1812-x
Khan K, Sharan K, Swarnkar G, Chakravarti B, Mittal M, Barbhuyan TK, China SP, Khan MP, Nagar GK, Yadav D, Dixit P (2013) Positive skeletal effects of cladrin, a naturally occurring dimethoxydaidzein, in osteopenic rats that were maintained after treatment discontinuation. Osteoporos Int 24:1455–1470. https://doi.org/10.1007/s00198-012-2121-8
Funding
Supporting Grants: Centre for Research in Anabolic Skeletal Targets in Health and Illness (ASTHI), Council of Scientific and Industrial Research (CSIR), National Medicinal Plants Board (NMPB) project number R&D/UP-01/2015, Ministry of AYUSH, Government of India.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Ravi Prakash, Tripti Mishra, Kapil Dev, Kriti Sharma, Jitendra Kuldeep, Aijaz Ahmad John, Alok Tripathi, Chetan Sharma, Kamal Ram Arya, Brijesh Kumar, Mohd Imran Siddiqi, Narender Tadigoppula, and Divya Singh have no conflicts of interest.
Human and Animal Rights and Informed Consent
The experimental procedure was performed according to the regulations and guidelines approved by the Institutional Animal Ethics Committee (IAEC), Council of Scientific and Industrial Research-Central Drug Research Institute (CPCSEA Registration number: 34/GO/REBI/S/99 dated 12.3.15; Approval reference number: IAEC/2012/70N/Renew 05 (214/16) dated 4.11.2016) and Council for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The CDRI communication number is 10203.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prakash, R., Mishra, T., Dev, K. et al. Phenanthrenoid Coelogin Isolated from Coelogyne cristata Exerts Osteoprotective Effect Through MAPK-Mitogen-Activated Protein Kinase Signaling Pathway. Calcif Tissue Int 109, 32–43 (2021). https://doi.org/10.1007/s00223-021-00818-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00818-3