Abstract
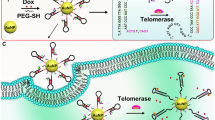
Telomerase is considered a valuable diagnostic and prognostic cancer biomarker. Accurate and reliable detection of telomerase activity is of great value in clinical diagnosis, screening of inhibitors, and therapeutics. Here, we developed a novel amplified fluorescence resonance energy transfer (FRET) nanoprobe for highly sensitive and reliable monitoring of intracellular telomerase activity. The nanoprobe (QDSA@DNA) was composed of a streptavidin-modified quantum dot (QDSA) which was functionalized with a telomerase primer sequence (TP) and Cy5-tagged signal switching sequence (SS) through biotin-streptavidin interaction. When the nanoprobe was assembled, the Cy5 was in close proximity to the QDSA, resulting in high FRET efficiency from the QDSA to Cy5. In the presence of telomerase, the TP could be extended to produce telomeric repeat units, which was complementary to the loop of SS. Thus, the SS could hybridize with elongated sequences to form a rigid double-stranded structure, which forced the Cy5 away from the surface of the QDSA, causing low FRET efficiency. Furthermore, due to the production of multiple repeat units by telomerase, multiple hairpin structures could be opened, yielding significant fluorescence ratio (FQDsa/FCy5) enhancement for sensing of telomerase activity. In this way, the combination of a FRET and target-assisted strategy in a nanoprobe improved the detection accuracy and amplified the detection signal, respectively. The QDSA@DNA nanoprobe also showed high selectivity, excellent nuclease stability, and good biocompatibility. More importantly, this nanoprobe was found to be an excellent platform for efficient monitoring of intracellular telomerase activity, providing a potential platform in tumor diagnosis and screening of telomerase-related inhibitors.
Graphical abstract







Similar content being viewed by others
References
Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–6.
Hammond PW, Cech TR. dGTP-dependent processivity and possible template switching of euplotes telomerase. Nucleic Acids Res. 1997;25(18):3698–704.
Baur JA, Zou Y, Shay JW, Wright WE. Telomere position effect in human cells. Science. 2001;292(5524):2075–7.
Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33(5):787–91.
Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–22.
Arndt GM, MacKenzie KL. New prospects for targeting telomerase beyond the telomere. Nat Rev Cancer. 2016;16:508–24.
Zhou XM, Xing D. Assays for human telomerase activity: progress and prospects. Chem Soc Rev. 2012;41(13):4643–56.
Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, et al. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–70.
Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–3.
Herbert BS, Hochreiter AE, Wright WE, Shay JW. Nonradioactive detection of telomerase activity using the telomeric repeat amplification protocol. Nat Protoc. 2006;1:1583–90.
Kim NW, Wu F. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 1997;25(13):2595–7.
Su D, Huang XY, Dong CQ, Ren JC. Quantitative determination of telomerase activity by combining fluorescence correlation spectroscopy with telomerase repeat amplification protocol. Anal Chem. 2017;90(1):1006–13.
Dong PF, Zhu LY, Huang J, Ren JJ, Lei JP. Electrocatalysis of cerium metal-organic frameworks for ratiometric electrochemical detection of telomerase activity. Biosens Bioelectron. 2019;138(1):111313.
Yu T, Zhao W, Xu JJ, Chen HY. A PCR-free colorimetric strategy for visualized assay of telomerase activity. Talanta. 2018;178(1):594–9.
Sharon E, Freeman R, Riskin M, Gil N, Tzfati Y, Willner I. Optical, electrical and surface plasmon resonance methods for detecting telomerase activity. Anal Chem. 2010;82(20):8390–7.
Wu QL, Liu ZJ, Su L, Han GM, Liu RY, Zhao J, et al. Sticky-flares for in situ monitoring of human telomerase RNA in living cells. Nanoscale. 2018;10(19):9386–92.
Chen XL, Deng YY, Cao GH, Liu XY, Gu T, Feng RY, et al. An ultrasensitive and point-of-care sensor for the telomerase activity detection. Anal Chim Acta. 2021;1146(15):61–9.
Yu T, Zhao W, Xu JJ, Chen HY. A PCR-free colorimetric strategy for visualized assay of telomerase activity. Talanta. 2018;178:594–9.
Geißler D, Hildebrandt N. Recent developments in Förster resonance energy transfer (FRET) diagnostics using quantum dots. Anal Bioanal Chem. 2016;408:4475–83.
Jin S, Wu C, Ying Y, Ying YB, Ye ZZ. Magnetically separable and recyclable bamboo-like carbon nanotube-based FRET assay for sensitive and selective detection of Hg2+. Anal Bioanal Chem. 2020;412:3779–86.
Yang YJ, Huang J, Yang XH, Quan K, Wang H, Ying L, et al. FRET nanoflares for intracellular mRNA detection: avoiding false positive signals and minimizing effects of system fluctuations. J Am Chem Soc. 2015;137(26):8340–3.
Hu J, Liu MH, Zhang CY. Construction of tetrahedral DNA-quantum dot nanostructure with the integration of multistep Forster resonance energy transfer for multiplex enzymes assay. ACS Nano. 2019;13(6):7191–201.
Tao Deng T, Peng YA, Zhang R, Jie Wang J, et al. Water-solubilizing hydrophobic ZnAgInSe/ZnS QDs with tumor-targeted cRGD-sulfobetaine-PIMA-histamine ligands via a self-assembly strategy for bioimaging. ACS Appl Mater Interfaces. 2017;9(13):11405–14.
Singh N, Charan S, Sanjiv K, Huang SH, Hsiao YH, et al. Synthesis of tunable and multifunctional Ni-doped near-infrared QDs for cancer cell targeting and cellular sorting. Bioconjugate Chem. 2012;23(3):421–30.
Singh VK, Mishra HS, Ali R, Umrao S, et al. In situ functionalized fluorescent WS2-QDs as sensitive and selective probe for Fe3+ and a detailed study of its fluorescence quenching. Appl Nano Mater. 2019;2(1):566–76.
Jiao XY, Zhou YB, Zhao D, Pang D, et al. An indirect ELISA-inspired dual-channel fluorescent immunoassay based on MPA-capped CdTe/ZnS QDs. Anal Bioanal Chem. 2019;411:5437–44.
Kaniyankandy S, Verma S. Role of core−shell formation in exciton confinement relaxation in dithiocarbamate-capped CdSe QDs. J Phys Chem Lett. 2017;8(14):3228–33.
Hildebrandt N, Spillmann CM, Pons T, Stewart MH, et al. Energy transfer with semiconductor quantum dot bioconjugates: a versatile platform for biosensing, energy harvesting, and other developing applications. Chem Rev. 2017;117(2):536–711.
Chen JR, Sun N, Chen HH, Zhang YT, Wang XL, Zhou ND. A FRET-based detection of N-acetylneuraminic acid using CdSe/ZnS quantum dot and exonuclease III-assisted recycling amplification strategy. Food Chem. 2022;367:130754.
Yang GH, Zhang Q, Ma L, Zheng YW, Tian F, Li HT, et al. Sensitive detection of telomerase activity in cells using a DNA-based fluorescence resonance energy transfer nanoprobe. Anal Chim Acta. 2020;1098(15):133–9.
Santos MCD, Algar WR, Medintz IL, Hildebrandt N. Quantum dots for förster resonance energy transfer (FRET). TrAC-Trend Anal Chem. 2020;125:115819.
Zhang Y, Li QN, Zhou KY, Xu QF, Zhang CY. Identification of specific N6-methyladenosine RNA demethylase FTO inhibitors by single-quantum-dot-based FRET nanosensors. Anal Chem. 2020;92(20):13936–44.
Hong M, Xu LD, Xue QW, Li L, Tang B. Fluorescence imaging of intracellular telomerase activity using enzyme-free signal amplification. Anal Chem. 2016;88(24):12177–82.
Qian RC, Ding L, Yan LW, Lin MF, Ju HX. Smart vesicle kit for in situ monitoring of intracellular telomerase activity using a telomerase-responsive probe. Anal Chem. 2014;86(17):8642–8.
Acknowledgements
The authors thank the College Central Laboratory, Changzhi Medical College for valuable help in our experiment.
Funding
This work is financially supported by the National Natural Science Foundation of China (31900587), the Natural Science Foundation for Young Scientists of Shanxi Province, China (201901D211476), the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi, China (2019L0663, 2019L0532), Outstanding Doctor Funding Award of Shanxi Province, China (SXYBKY2020001), Doctoral Scientific Research Foundation of Changzhi Medical College, China (BS201919), and the Innovation Team (CX201904) of Changzhi Medical College.
Author information
Authors and Affiliations
Contributions
Dandan Ma: funding acquisition, project administration, investigation, visualization, writing—original draft. Huiyun Bai: conceptualization, methodology, data curation. Junbo Li: investigation. Yintao Li: investigation. Lihua Song: investigation. Jingping Zheng: conceptualization. Congxiu Miao: resources, supervision, writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Research involving human participants and/or animals
The authors declare that no human participants and/or animals were involved in this research.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, D., Bai, H., Li, J. et al. A ratiometric fluorescent nanoprobe for signal amplification monitoring of intracellular telomerase activity. Anal Bioanal Chem 414, 1891–1898 (2022). https://doi.org/10.1007/s00216-021-03823-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03823-5