Abstract
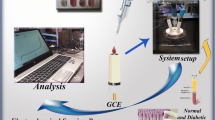
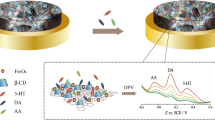
Serotonin (5-HT) levels have been associated with several exclusively metabolic disorders. Herein, a new approach for 5-HT level as a novel biomarker of diabetes mellitus is considered using a simple nanocomposite and HPLC method. Reduced graphene oxide (rGO) comprising gold nanoparticles (AuNPs) was decorated with 18-crown-6 (18.Cr.6) to fabricate a simple nanocomposite (rGO-AuNPs-18.Cr.6). The nanocomposite was positioned on a glassy carbon electrode (GCE) to form an electrochemical sensor for the biomarker 5-HT in the presence of L-tryptophan (L-Trp), dopamine (DA), ascorbic acid (AA), urea, and glucose. The nanocomposite exhibited efficient catalytic activity for 5-HT detection by square-wave voltammetry (SWV). The proposed sensor displayed high selectivity, excellent reproducibility, notable anti-interference ability, and long-term stability even after 2 months. SWV defined a linear range of 5-HT concentration from 0.4 to 10 μg L−1. A diabetic animal model (diabetic zebrafish model) was then applied to investigate 5-HT as a novel biomarker of diabetes. A limit of detection (LOD) of about 0.33 μg L−1 was found for the diabetic group and 0.15 μg L−1 for the control group. The average levels of 5-HT obtained were 9 and 2 μg L−1 for control and diabetic groups, respectively. The recovery, relative standard deviation (RSD), and relative error (RE) were found to be about 97%, less than 2%, and around 3%, respectively. The significant reduction in 5-HT level in the diabetic group compared to the control group proved that the biomarker 5-HT can be applied for the early diagnosis of diabetes mellitus.
Graphical abstract













Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Xue C, Wang X, Zhu W, Han Q, Zhu C, Hong J, et al. Electrochemical serotonin sensing interface based on double-layered membrane of reduced graphene oxide/polyaniline nanocomposites and molecularly imprinted polymers embedded with gold nanoparticles. Sensors Actuators B Chem. 2014;196:57–63.
Kema IP, Meijer WG, Meiborg G, Ooms B, Willemse PHB, de Vries EGE. Profiling of tryptophan-related plasma indoles in patients with carcinoid tumors by automated, on-line, solid-phase extraction and HPLC with fluorescence detection. Clin Chem. 2001;47(10):1811–20.
Liang W, Rong Y, Fan L, Zhang C, Dong W, Li J, et al. Simultaneous electrochemical sensing of serotonin, dopamine and ascorbic acid by using a nanocomposite prepared from reduced graphene oxide, Fe3O4 and hydroxypropyl-β-cyclodextrin. Microchim Acta. 2019;186(12):751.
Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol. 2008;48(4):666–75.
Rieder R, Wisniewski PJ, Alderman BL, Campbell SC. Microbes and mental health: a review. Brain Behav Immun. 2017;66:9–17.
Cataldo Bascuñan LR, Lyons C, Bennet H, Artner I, Fex M. Serotonergic regulation of insulin secretion. Acta Physiol. 2019;225(1):e13101.
Tsunoda M, Takezawa K, Santa T, Imai K. Simultaneous automatic determination of catecholamines and their 3-O-methyl metabolites in rat plasma by high-performance liquid chromatography using peroxyoxalate chemiluminescence reaction. Anal Biochem. 1999;269(2):386–92.
Panholzer TJ, Beyer J, Lichtwald K. Coupled-column liquid chromatographic analysis of catecholamines, serotonin, and metabolites in human urine. Clin Chem. 1999;45(2):262–8.
Tsai H, Whang C. Capillary electrophoresis of monoamines and catechol with indirect chemiluminescence detection. Electrophoresis. 1999;20(12):2533–8.
Yoshitake T, Fujino K, Kehr J, Ishida J, Nohta H, Yamaguchi M. Simultaneous determination of norepinephrine, serotonin, and 5-hydroxyindole-3-acetic acid in microdialysis samples from rat brain by microbore column liquid chromatography with fluorescence detection following derivatization with benzylamine. Anal Biochem. 2003;312(2):125–33.
Cao J, Murch SJ, O’Brien R, Saxena PK. Rapid method for accurate analysis of melatonin, serotonin and auxin in plant samples using liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2006;1134(1–2):333–7.
Khoshnevisan K, Honarvarfard E, Torabi F, Maleki H, Baharifar H, Faridbod F, et al. Electrochemical detection of serotonin: a new approach. Clin Chim Acta. 2020;501:112–9.
Sharma S, Singh N, Tomar V, Chandra R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens Bioelectron. 2018;107:76–93.
Khoshnevisan K, Maleki H, Honarvarfard E, Baharifar H, Gholami M, Faridbod F, et al. Nanomaterial based electrochemical sensing of the biomarker serotonin: a comprehensive review. Microchim Acta. 2019;186(1):49.
Liu YL, Chen Y, Fan WT, Cao P, Yan J, Zhao XZ, et al. Mechanical distension induces serotonin release from intestine as revealed by stretchable electrochemical sensing. Angew Chem Int Ed. 2020;59(10):4075–81.
Khan MZH, Liu X, Tang Y, Zhu J, Hu W, Liu X. A glassy carbon electrode modified with a composite consisting of gold nanoparticle, reduced graphene oxide and poly(L-arginine) for simultaneous voltammetric determination of dopamine, serotonin and L-tryptophan. Microchim Acta. 2018;185(9):3–12.
Mahato K, Purohit B, Bhardwaj K, Jaiswal A, Chandra P. Novel electrochemical biosensor for serotonin detection based on gold nanorattles decorated reduced graphene oxide in biological fluids and in vitro model. Biosens Bioelectron. 2019;142(July):111502.
Adumitrăchioaie A, Tertiș M, Suciu M, Graur F, Cristea C. A novel immunosensing platform for serotonin detection in complex real samples based on graphene oxide and chitosan. Electrochim Acta. 2019;311:50–61.
Panneer Selvam S, Yun K. A self-assembled silver chalcogenide electrochemical sensor based on rGO-Ag2Se for highly selective detection of serotonin. Sensors Actuators B Chem. 2020;302(May 2019):127161.
Özel RE, Wallace KN, Andreescu S. Chitosan coated carbon fiber microelectrode for selective in vivo detection of neurotransmitters in live zebrafish embryos. Anal Chim Acta. 2011;695(1–2):89–95.
Atta NF, Ahmed YM, Galal A. Layered-designed composite sensor based on crown ether/Nafion®/polymer/carbon nanotubes for determination of norepinephrine, paracetamol, tyrosine and ascorbic acid in biological fluids. J Electroanal Chem. 2018;828(September):11–23.
Mukdasai S, Poosittisak S, Ngeontae W, Srijaranai S. A highly sensitive electrochemical determination of l-tryptophan in the presence of ascorbic acid and uric acid using in situ addition of tetrabutylammonium bromide on the ß-cyclodextrin incorporated multi-walled carbon nanotubes modified electrode. Sensors Actuators B Chem. 2018;272:518–25.
Atta NF, Ahmed YM, Galal A. Electrochemical determination of neurotransmitters at crown ether modified carbon nanotube composite: application for sub-nano-sensing of serotonin in human serum. Electroanalysis. 2019;31(7):1204–14.
Pedersen CJ. The discovery of crown ethers (Noble lecture). Angew Chem Int Ed Eng. 1988;27(8):1021–7.
Lai G-S, Zhang H-L, Jin C-M. Electrocatalysis and voltammetric determination of dopamine at a calix[4]arene crown-4 ether modified glassy carbon electrode. Electroanalysis. 2007;19(4):496–501.
Muzzalupo R, Nicoletta FP, Trombino S, Cassano R, Iemma F, Picci N. A new crown ether as vesicular carrier for 5-fluoruracil: synthesis, characterization and drug delivery evaluation. Colloids Surf B: Biointerfaces. 2007;58(2):197–202.
Zhang W, Liu F, Li Q, Shou Q, Cheng J, Zhang L, et al. Transition metal oxide and graphene nanocomposites for high-performance electrochemical capacitors. Phys Chem Chem Phys. 2012;14(47):16331–7.
Dinesh B, Veeramani V, Chen S-M, Saraswathi R. In situ electrochemical synthesis of reduced graphene oxide-cobalt oxide nanocomposite modified electrode for selective sensing of depression biomarker in the presence of ascorbic acid and dopamine. J Electroanal Chem. 2017;786:169–76.
Robinson R. Serotonin’s role in the pancreas revealed at last. PLoS Biol 2009;7(10):e1000227.
Oh C-M, Park S, Kim H. Serotonin as a new therapeutic target for diabetes mellitus and obesity. Diabetes Metab J. 2016;40(2):89–98.
Zang L, Shimada Y, Nishimura N. Development of a novel zebrafish model for type 2 diabetes mellitus. Sci Rep. 2017;7(1):1–11.
Intine RV, Olsen AS, Sarras MP Jr. A zebrafish model of diabetes mellitus and metabolic memory. J Vis Exp. 2013;72:e50232.
Capiotti KM, Antonioli R, Kist LW, Bogo MR, Bonan CD, Da Silva RS. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp Biochem Physiol B Biochem Mol Biol. 2014;171(1):58–65.
Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255(1):12–29.
Njagi J, Ball M, Best M, Wallace KN, Andreescu S. Electrochemical quantification of serotonin in the live embryonic zebrafish intestine. Anal Chem. 2010;82(5):1822–30.
Khoshnevisan K, Torabi F, Baharifar H, Sajjadi-Jazi SM, Afjeh MS, Faridbod F, et al. Determination of the biomarker L-tryptophan level in diabetic and normal human serum based on an electrochemical sensing method using reduced graphene oxide/gold nanoparticles/18-crown-6. Anal Bioanal Chem. 2020;1–3. https://doi.org/10.1007/s00216-020-02598-5.
Li J, Jiang J, Xu Z, Liu M, Tang S, Yang C, et al. Facile synthesis of Pd−cu@Cu2O/N-RGO hybrid and its application for electrochemical detection of tryptophan. Electrochim Acta. 2018;260:526–35.
Khan MZH, Liu X, Tang Y, Zhu J, Hu W, Liu X. A glassy carbon electrode modified with a composite consisting of gold nanoparticle, reduced graphene oxide and poly(L-arginine) for simultaneous voltammetric determination of dopamine, serotonin and L-tryptophan. Microchim Acta. 2018;185(9):439.
Thomas J, Khanam R, Vohora D. A validated HPLC-UV method and optimization of sample preparation technique for norepinephrine and serotonin in mouse brain. Pharm Biol. 2015;53(10):1539–44.
Komura J, Sakamoto M. Determination of biogenic amines and their metabolites in regional tissue of mouse brain by high performance liquid chromatography using an ultraviolet detector. J Liq Chromatogr. 1990;13(7):1291–9.
Song J, Yang C, Ma J, Han Q, Ran P, Fu Y. Voltammetric chiral discrimination of tryptophan using a multilayer nanocomposite with implemented amino-modified $β$-cyclodextrin as recognition element. Microchim Acta. 2018;185(4):230.
Jia X, Chen X, Han J, Ma J, Ma Z. Triple signal amplification using gold nanoparticles, bienzyme and platinum nanoparticles functionalized graphene as enhancers for simultaneous multiple electrochemical immunoassay. Biosens Bioelectron. 2014;53:65–70.
Nehru L, Chinnathambi S, Fazio E, Neri F, Leonardi GS, Bonavita A, et al. Electrochemical sensing of serotonin by a modified MnO2-graphene electrode. Vol. 10, Biosensors. 2020;10(4):33.
Orzari LO, Cristina de Freitas R, Aparecida de Araujo Andreotti I, Gatti A, Janegitz BC. A novel disposable self-adhesive inked paper device for electrochemical sensing of dopamine and serotonin neurotransmitters and biosensing of glucose. Biosens Bioelectron. 2019;138:111310.
Sun D, Li H, Li M, Li C, Dai H, Sun D, et al. Electrodeposition synthesis of a NiO/CNT/PEDOT composite for simultaneous detection of dopamine, serotonin, and tryptophan. Sensors Actuators B Chem. 2018;259:433–42.
Ran G, Chen C, Gu C. Serotonin sensor based on a glassy carbon electrode modified with multiwalled carbon nanotubes, chitosan and poly(p-aminobenzenesulfonate). Microchim Acta. 2015;182(7–8):1323–8.
Pietraszek MH, Takada Y, Takada A, Fujita M, Watanabe I, Taminato A, et al. Blood serotonergic mechanisms in type 2 (non-insulin-dependent) diabetes mellitus. Thromb Res. 1992;66(6):765–74.
Hara K, Hirowatari Y, Shimura Y, Takahashi H. Serotonin levels in platelet-poor plasma and whole blood in people with type 2 diabetes with chronic kidney disease. Diabetes Res Clin Pract 2011;94(2):167–71.
Barradas MA, Gill DS, Fonseca VA, Mikhailidis DP, Dandona P. Intraplatelet serotonin in patients with diabetes mellitus and peripheral vascular disease. Eur J Clin Investig. 1988;18(4):399–404.
Acknowledgments
This work was supported by the Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences (Grant No. 1396-01-100-2249). The authors would like to express special thanks to Dr. Farshad Sharifi for his valuable insight.
Authorship contribution statement
K. Khoshnevisan: conceptualization, formal analysis, investigation, methodology, resources, design, software, validation, visualization, writing original draft, writing/review and editing. H. Baharifar: data curation, investigation, methodology, resources, software, validation, visualization, writing original draft. F. Torabi: data curation, software, investigation, validation, resources. M. Sadeghi Afjeh: investigation, data curation, methodology, resources, validation, software. H. Maleki: data curation, design, software, investigation. E. Honarvarfard: investigation, writing/review and editing, software, design. H. Mohammadi: formal analysis, resources, investigation. SM. Sajjadi-Jazi: investigation, data curation, resources, writing original draft. S. Mahmoudi-Kohan: investigation, data curation, software. F. Faridbod: conceptualization, supervision, investigation, resources, visualization. B. Larijani: funding acquisition, project administration, resources, supervision. FS: investigation, conceptualization, visualization. R. Faridi Majidi: investigation, visualization, conceptualization. M.R. Khorramizadeh: conceptualization, funding acquisition, investigation, project administration, resources, supervision, visualization, writing/review and editing.
Funding
This study was financially supported by the Iran National Committee for Medical Research Development (NIMAD), Tehran, Iran (Grant No. 971040).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of NIMAD.
Consent for publication
All the authors have approved the manuscript and agreed with submission.
Declaration of competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khoshnevisan, K., Baharifar, H., Torabi, F. et al. Serotonin level as a potent diabetes biomarker based on electrochemical sensing: a new approach in a zebra fish model. Anal Bioanal Chem 413, 1615–1627 (2021). https://doi.org/10.1007/s00216-020-03122-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-03122-5