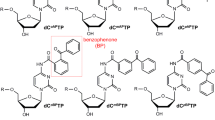
Abstract
Ultraviolet (UV) radiation could induce pyrimidine-related dimeric lesions in genomic DNA. Though the cyclobutane pyrimidine dimers (CPDs) are the most abundant UV-induced lesions, the pyrimidine (6-4) pyrimidone photoproducts (6-4PPs) may have more serious, potentially lethal, and mutagenic effects. It is important to have 6-4PP-containing oligodeoxynucleotides to be prepared for studying their adverse biological effects. Here, we developed a UV-irradiated water droplet method for the preparation of a biotinylated, 6-4PP-containing 10-mer oligodeoxynucleotide. By the use of HPLC purification and enrichment twice, the final yield is estimated to be about 8.1%. In contrast, without applying droplet technique, the direct UV irradiation against oligonucleotide-containing aqueous solution, the product yield is very low. The enzymatic hydrolyzation of the obtained product shows a 6-4PP characteristic ion transition of 545.12 → 432.13 in negative ion mode UHPLC-Q-TOF/MS. The established procedure for the preparation of 6-4PP-containing oligonucleotides is convenient with an improved yield.

ᅟ




Similar content being viewed by others
References
Sinha RP, Häder DP. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1(4):225–36.
Douki T, Reynaudangelin A, Jean Cadet A, Sage E. Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry. 2003;42(30):9221–6.
Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res. 2005;571(1):3–17.
Heil K, Pearson D, Carell T. Chemical investigation of light induced DNA bipyrimidine damage and repair. Chem Soc Rev. 2011;40(8):4271–8.
Li X, Eriksson LA. Influence of C5-methylation of cytosine on the formation of cyclobutane pyrimidine dimers. Chem Phys Lett. 2005;401(1):99–103.
Batista L, Kaina B, Meneghini R, Menck C. How DNA lesions are turned into powerful killing structures: insights from UV-induced apoptosis. Mutat Res. 2009;681(2):197–208.
Matsunaga T, Hieda K, Nikaido O. Wavelength dependent formation of thymine dimers and (6-4) photoproducts in DNA by monochromatic ultraviolet light ranging from 150 to 365 nm. Photochem Photobiol. 2010;54(3):403–10.
Hatton DH, Mitchell DL, Strickland PT, Johnson RT. Enhanced photoproduct repair: its role in the DNA damage-resistance phenotype of human malignant melanoma cells. Cancer Res. 1995;55(1):181–9.
Mitchell DL, Rosenstein BS. The use of specific radioimmunoassay to determine action spectra for the photolysis of (6-4) photoproducts. Photochem Photobiol. 2010;45(S1):781–6.
Cleaver JE, Charles WC, Mcdowell ML, Sadinski WJ, Mitchell DL. Overexpression of the XPA repair gene increases resistance to ultraviolet radiation in human cells by selective repair of DNA damage. Cancer Res. 1995;55(24):6152–60.
Chouinard N, Therrien JP, Mitchell DL, Robert M, Drouin R, Rouabhia M. Repeated exposures of human skin equivalent to low doses of ultraviolet-B radiation lead to changes in cellular functions and accumulation of cyclobutane pyrimidine dimers. Biochem Cell Biol. 2001;79(4):507–15.
Kong B, Yang C, Wu D, An L, Ran F, Yan L, et al. Affinity maturation of an antibody for the UV-induced DNA lesions 6,4 pyrimidine-pyrimidones. Appl Microbiol Biotechnol. 2018:1–16.
Iwai S. Preparation of oligodeoxyribonucleotides containing the pyrimidine(6-4) pyrimidone photoproduct by using a dinucleotide building block. 2013;Chapter 4(Chapter 4):Unit4.56.
Franklin WA, Lo KM, Haseltine WA. Alkaline lability of fluorescent photoproducts produced in ultraviolet light-irradiated DNA. J Biol Chem. 1982;257(22):13535–43.
Iwai S, Mizukoshi T, Fujiwara Y, Masutani C, Hanaoka F, Hayakawa Y. Benzimidazolium triflate-activated synthesis of (6-4) photoproduct-containing oligonucleotides and its application. Nucleic Acids Res. 1999;27(11):2299–303.
Wu D, Lai W, Lyu C, Hang H, Wang H. UHPLC-Q-TOF/MS detection of UV-induced TpT dimeric lesions in genomic DNA. J Chromatogr B Anal Technol Biomed Life Sci. 2018;1096:135–42. https://doi.org/10.1016/j.jchromb.2018.08.015.
Al-Hussein A, Gieseler H. Investigation of histidine stabilizing effects on LDH during freeze-drying. J Pharm Sci. 2013;102(3):813–26.
Courdavault S, Baudouin C, Charveron M, Canguilhem B, Favier A, Cadet J, et al. Repair of the three main types of bipyrimidine DNA photoproducts in human keratinocytes exposed to UVB and UVA radiations. DNA Repair. 2005;4(7):836–44.
Douki T, Court M, Sauvaigo S, Odin F, Cadet J. Formation of the main UV-induced thymine dimeric lesions within isolated and cellular DNA as measured by high performance liquid chromatography-tandem mass spectrometry. J Biol Chem. 2000;275(16):11678.
Ransom M, Bryan DS, Hesselberth JR. High-resolution mapping of modified DNA nucleobases using excision repair enzymes. Genome Res. 2014;24(9):1534.
Funding
This work is supported by the National Natural Science Foundation of China (21435008, 9174321, and 21527901), Sanming Project of Medicine in Shenzhen (SZsm201811070), the Key Research Program of Frontier Sciences, CAS (QYZDJ-SSW-DQC017), and the K.C. Wong Education Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection New Insights into Analytical Science in China with guest editors Lihua Zhang, Hua Cui, and Qiankun Zhuang.
Rights and permissions
About this article
Cite this article
Wu, D., Zhang, N., Kong, B. et al. Synthesis and purification of biotinylated oligodeoxynucleotides containing single TpT dimeric pyrimidine (6-4) pyrimidone lesion. Anal Bioanal Chem 411, 4123–4129 (2019). https://doi.org/10.1007/s00216-018-01572-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-01572-6