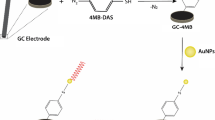
Abstract
A new type of magnetic nanoparticles (MNPs), as the absorbents of bisphenol A (BPA), was prepared by functionalization of Fe3O4@SiO2 with BPA-specific aptamer in this work. ssDNA aptamer was immobilized on the Fe3O4@SiO2 surface through biotin-avidin interactions, playing a role of the specific probe for BPA. The resultant materials (Apt-MNPs) exhibited outstanding magnetic responsibility and can be separated efficiently by the magnetic field. Experimental results also showed that Apt-MNPs had large adsorption capacity and high competitive selectivity for the targeted compound BPA. Furthermore, Apt-MNPs were adopted as the specific absorbents to extract and enrich BPA from human serum and urine samples. Therefore, an efficient detection method of BPA was developed in combination with high-performance liquid chromatography (HPLC). The linearity of the method was over a range of 5–10,000 ng mL−1 with a correlation coefficient of 0.99997, and the limit of detections (LODs) for serum and urine were 2.0 and 1.0 ng mL−1, respectively. The recoveries of BPA in the spiked human serum and urine samples were 90.8 ± 7.3% (RSD) and 92.3 ± 1.5%, respectively. Our results demonstrated that Apt-MNPs were high-performance adsorbents for extracting and enriching BPA, resulting in fast and efficient detection of BPA in serum and urine samples.

Aptamer-MNPs were effective for BPA separation from serum and urine








Similar content being viewed by others
References
Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–86.
Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. 2010;44:1458–63.
Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003-2006. Environ Health Perspect. 2011;119:390–6.
Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH, et al. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxidative Med Cell Longev. 2012;2012:194829.
Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010;1:146–55.
Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab. 2011;96:3822–6.
Liu J, Yu P, Qian W, Li Y, Zhao J, Huan F, et al. Perinatal bisphenol A exposure and adult glucose homeostasis: identifying critical windows of exposure. PLoS One. 2013;8:e64143.
Teppala S, Madhavan S, Shankar A. Bisphenol A and metabolic syndrome: results from NHANES. Int J Endocrinol. 2012;2012:598180.
Bodin J, Bolling AK, Samuelsen M, Becher R, Lovik M, Nygaard UC. Long-term bisphenol A exposure accelerates insulitis development in diabetes-prone NOD mice. Immunopharmcol Immunotoxicol. 2013;35:349–58.
Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect. 2012;120:1297–300.
Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224.
Sun F, Kang L, Xiang X, Li H, Luo X, Luo R, et al. Recent advances and progress in the detection of bisphenol A. Anal Bioanal Chem. 2016;408:6913–27.
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–22.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–10.
Moghaddam A, Lobersli I, Gebhardt K, Braunagel M, Marvik OJ. Selection and characterisation of recombinant single-chain antibodies to the hapten aflatoxin-B1 from naive recombinant antibody libraries. J Immunol Methods. 2001;254:169–81.
Jo M, Ahn JY, Lee J, Lee S, Hong SW, Yoo JW, et al. Development of single-stranded DNA aptamers for specific bisphenol a detection. Oligonucleotides. 2011;21:85–91.
Lee EH, Lim HJ, Lee SD, Son A. Highly sensitive detection of bisphenol A by NanoAptamer assay with truncated aptamer. ACS Appl Mater Interfaces. 2017;9:14889–98.
Li Y, Xu J, Wang L, Huang Y, Guo J, Cao X, et al. Aptamer-based fluorescent detection of bisphenol A using nonconjugated gold nanoparticles and CdTe quantum dots. Sensor Actuators B Chem. 2016;222:815–22.
Beiranvand ZS, Abbasi AR, Dehdashtian S, Karimi Z, Azadbakht A. Aptamer-based electrochemical biosensor by using Au-Pt nanoparticles, carbon nanotubes and acriflavine platform. Anal Biochem. 2017;518:35–45.
He MQ, Wang K, Wang J, Yu YL, He RHA. Sensitive aptasensor based on molybdenum carbide nanotubes and label-free aptamer for detection of bisphenol a. Anal Bioanal Chem. 2017;409:1797–803.
Guo X, Wu S, Duan N, Wang Z. Mn(2+)-doped NaYF4:Yb/Er upconversion nanoparticle-based electrochemiluminescent aptasensor for bisphenol A. Anal Bioanal Chem. 2016;408:3823–31.
Ragavan KV, Rastogi NK, Thakur MS. Sensors and biosensors for analysis of bisphenol-A. TrAC Trends Anal Chem. 2013;52:248–60.
Marks HL, Pishko MV, Jackson GW, Cote GL. Rational design of a bisphenol A aptamer selective surface-enhanced Raman scattering nanoprobe. Anal Chem. 2014;86:11614–9.
Zhu Y, Zhou C, Yan X, Yan Y, Wang Q. Aptamer-functionalized nanoporous gold film for high-performance direct electrochemical detection of bisphenol A in human serum. Anal Chim Acta. 2015;883:81–9.
Lin X, Cheng C, Terry P, Chen J, Cui H, Wu J. Rapid and sensitive detection of bisphenol a from serum matrix. Biosens Bioelectron. 2017;91:104–9.
Zhang T, Sun H, Kannan K. Blood and urinary bisphenol A concentrations in children, adults, and pregnant women from china: partitioning between blood and urine and maternal and fetal cord blood. Environ Sci Technol. 2013;47:4686–94.
Olsen L, Lampa E, Birkholz DA, Lind L, Lind PM. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the prospective investigation of the vasculature in Uppsala seniors (PIVUS). Ecotoxicol Environ Saf. 2012;75:242–8.
Kim YS, Gu MB. Advances in aptamer screening and small molecule aptasensors. Adv Biochem Eng Biotechnol. 2014;140:29–67.
Wang H, Zhang A, Wang W, Zhang M, Liu H, Wang X. Separation and determination of triclosan and bisphenol A in water, beverage, and urine samples by dispersive liquid-liquid microextraction combined with capillary zone electrophoresis-UV detection. J AOAC Int. 2013;96:459–65.
Moors S, Blaszkewicz M, Bolt HM, Degen GH. Simultaneous determination of daidzein, equol, genistein and bisphenol A in human urine by a fast and simple method using SPE and GC-MS. Mol Nutr Food Res. 2007;51:787–98.
Mao L, Sun C, Zhang H, Li Y, Wu D. Determination of environmental estrogens in human urine by high performance liquid chromatography after fluorescent derivatization with p-nitrobenzoyl chloride. Anal Chim Acta. 2004;522:241–6.
Cobellis L, Colacurci N, Trabucco E, Carpentiero C, Grumetto L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed Chromatogr. 2009;23:1186–90.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21107083), Wenzhou Public Welfare Science and Technology Project (S20150019, Y20160024), Zhejiang Medical and Health Science & Technology Project (2018PY032), Health Bureau of Zhejiang Province (11-CX29), Zhejiang Province College Students Science and Technology Innovation Project (Xinmiao Talent Program) (2015R413065), and Research project for College Students of Wenzhou Medical University (wyx2016101096).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
Human urine sample used in this study was collected from a healthy volunteer. This work was performed with the written informed consent of the healthy volunteer. The studies were approved by The Ethics Committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, and performed in accordance of the ethical standards.
Rights and permissions
About this article
Cite this article
Su, Y., Shao, C., Huang, X. et al. Extraction and detection of bisphenol A in human serum and urine by aptamer-functionalized magnetic nanoparticles. Anal Bioanal Chem 410, 1885–1891 (2018). https://doi.org/10.1007/s00216-017-0801-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0801-0