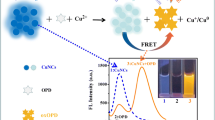
Abstract
Nitroaromatic compounds, for example trinitrotoluene and 2,4,6-trinitrophenol (TNP), are well-known primary constituents of many unexploded landmines worldwide. These compounds are recognized as environmental contaminants and as toxic to living organisms. Therefore, Förster resonance energy transfer (FRET) for TNP detection was developed on the basis of spectral overlap between the fluorescence spectrum of copper nanoclusters (CuNCs) and absorption spectrum of TNP (the calculated Förster distance R 0 of the donor CuNCs and the acceptor TNP is 2.8 nm). Water-soluble fluorescent CuNCs capped with bovine serum albumin have fluorescence emission from 350 nm to 500 nm with maximum fluorescence emission at 400 nm, which overlaps with the absorption spectra of TNP from 350 nm to 450 nm. Inspired by FRET structures, an unprecedented energy-donor-and-acceptor pair of fluorescent CuNCs and TNP is developed in this work. Fluorescence of CuNCs is quenched in the presence of TNP as a result of FRET from fluorescent CuNCs to TNP. Therefore a fluorescence quenching method for the determination of TNP is developed. It achieves TNP detection from 0.8 μmol L−1 to 100 μmol L−1, with response within 1 min and with good selectivity compared with that for other nitroaromatic compounds, including 2,4-dinitrotoluene, p-nitrotoluene, and nitrobenzene, and phenol.

Förster resonance-energy-transfer detection of 2,4,6-trinitrophenol using copper nanoclusters was first developed. It achieves TNP detection from 0.8 mmol L−1 to 100 mmol L−1 within 1 min with good selectivity compared with other nitroaromatic compounds






Similar content being viewed by others
References
Xu B, Wu X, Li H, Tong H, Wang L (2011) Selective detection of TNT and picric acid by conjugated polymer film sensors with donor–acceptor architecture. Macromolecules 44:5089–5092
Ma Y, Li H, Peng S, Wang L (2012) Highly selective and sensitive fluorescent paper sensor for nitroaromatic explosive detection. Anal Chem 84:8415–8421
Bhalla V, Gupta A, Kumar M, Rao DS, Prasad SK (2013) Self-assembled pentacenequinone derivative for trace detection of picric acid. ACS Appl Mater Inter 5:672–679
Li X-G, Liao Y, Huang M-R, Strong V, Kaner RB (2013) Ultra-sensitive chemosensors for Fe(iii) and explosives based on highly fluorescent oligofluoranthene. Chem Sci 4:1970–1978
Babaee S, Beiraghi A (2010) Micellar extraction and high performance liquid chromatography-ultra violet determination of some explosives in water samples. Anal Chim Acta 662:9–13
Mu R, Shi H, Yuan Y, Karnjanapiboonwong A, Burken JG, Ma Y (2012) Fast separation and quantification method for nitroguanidine and 2,4-dinitroanisole by ultrafast liquid chromatography-tandem mass spectrometry. Anal Chem 84:3427–3432
Peng Y, Zhang AJ, Dong M, Wang YW (2011) A colorimetric and fluorescent chemosensor for the detection of an explosive–2,4,6-trinitrophenol (TNP). Chem Commun 47:4505–4507
Dong M, Wang YW, Zhang AJ, Peng Y (2013) Colorimetric and fluorescent chemosensors for the detection of 2,4,6-trinitrophenol and investigation of their co-crystal structures. Chem Asian J 8:1321–1330
Riskin M, Tel-Vered R, Bourenko T, Granot E, Willner I (2008) Imprinting of molecular recognition sites through electropolymerization of functionalized Au nanoparticles: Development of an electrochemical TNT sensor based on π-donor-acceptor interactions. J Am Chem Soc 130:9726–9733
An Z-F, Zheng C, Chen R-F, Yin J, Xiao J-J, Shi H-F, Tao Y, Qian Y, Huang W (2012) Exceptional blueshifted and enhanced aggregation-induced emission of conjugated asymmetric triazines and their applications in superamplified detection of explosives. Chem-Eur J 18:15655–15661
Xu Y, Li B, Li W, Zhao J, Sun S, Pang Y (2013) "ICT-not-quenching" near infrared ratiometric fluorescent detection of picric acid in aqueous media. Chem Commun 49:4764–4766
Gonzalez CM, Iqbal M, Dasog M, Piercey DG, Lockwood R, Klapotke TM, Veinot JG (2014) Detection of high-energy compounds using photoluminescent silicon nanocrystal paper based sensors. Nanoscale 6:2608–2612
Cheng S, Dou J, Wang W, Chen C, Hua L, Zhou Q, Hou K, Li J, Li H (2013) Dopant-assisted negative photoionization ion mobility spectrometry for sensitive detection of explosives. Anal Chem 85:319–326
Shankaran D, Gobi K, Matsumoto K, Imato T, Toko K, Miura N (2004) Highly sensitive surface plasmon resonance immunosensor for parts-per-trillion level detection of 2,4,6-trinitrophenol. Sensor Actuat B-Chem 100:450–454
Ko H, Chang S, Tsukruk VV (2009) Porous substrates for label-free molecular level detection of nonresonant organic molecules. ACS Nano 3:181–188
Venkatramaiah N, Kumar S, Patil S (2012) Fluoranthene based fluorescent chemosensors for detection of explosive nitroaromatics. Chem Commun 48:5007–5009
Goswami N, Giri A, Bootharaju MS, Xavier PL, Pradeep T, Pal SK (2011) Copper quantum clusters in protein matrix: potential sensor of Pb2+ ion. Anal Chem 83:9676–9680
Xie J, Zheng Y, Ying JY (2009) Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc 131:888–889
Hu L, Yuan Y, Zhang L, Zhao J, Majeed S, Xu G (2013) Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal Chim Acta 762:83–86
Guo C, Irudayaraj J (2011) Fluorescent Ag clusters via a protein-directed approach as a Hg(II) ion sensor. Anal Chem 83:2883–2889
Chen T, Hu Y, Cen Y, Chu X, Lu Y (2013) A dual-emission fluorescent nanocomplex of gold-cluster-decorated silica particles for live cell imaging of highly reactive oxygen species. J Am Chem Soc 135:11595–11602
Zhang P, Yang XX, Wang Y, Zhao NW, Xiong ZH, Huang CZ (2014) Rapid synthesis of highly luminescent and stable Au20 nanoclusters for active tumor-targeted imaging in vitro and in vivo. Nanoscale 6:2261–2269
Dey N, Samanta SK, Bhattacharya S (2013) Selective and efficient detection of nitro-aromatic explosives in multiple media including water, micelles, organogel, and solid support. ACS Appl Mater Inter 5:8394–8400
Huynh TP, Sosnowska M, Sobczak JW, Kc CB, Nesterov VN, D'Souza F, Kutner W (2013) Simultaneous chronoamperometry and piezoelectric microgravimetry determination of nitroaromatic explosives using molecularly imprinted thiophene polymers. Anal Chem 85:8361–8368
Bhalla V, Gupta A, Kumar M (2012) Fluorescent nanoaggregates of pentacenequinone derivative for selective sensing of picric acid in aqueous media. Org Lett 14:3112–3115
Nagarkar SS, Desai AV, Ghosh SK (2014) Fluorescent metal-organic framework for highly selective detection of nitro explosive in aqueous phase. Chem Commun 50:8915–8918
Dinda D, Gupta A, Shaw BK, Sadhu S, Saha SK (2014) ACS Appl Mater Interfaces 6:10722–10728
Descalzo AB, Somoza C, Moreno-Bondi MC, Orellana G (2013) Anal Chem 85:5316–5320
Acknowledgments
This work was supported by undergraduate training programs for innovation and entrepreneurship of Sichuan provincial education department (No. 201410644028), major project of Sichuan provincial education department (No. 13ZA0101), scientific research fund of Sichuan provincial education department (No. 12ZB315), and project of Dazhou municipal science and technology bureau application foundation (No. JCYJ1119).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, X., Huang, X. & Wu, D. Förster resonance-energy-transfer detection of 2,4,6-trinitrophenol using copper nanoclusters. Anal Bioanal Chem 407, 4607–4613 (2015). https://doi.org/10.1007/s00216-015-8657-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8657-7