Abstract
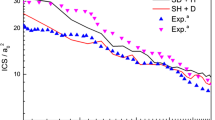
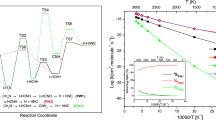
The \(\hbox {CN} + \hbox {H}_{2}\) reaction was investigated by considering the two possible channels, \(\hbox {H} + \hbox {HCN}\) and \(\hbox {H} + \hbox {HNC}\), taking into account the isotopic effects and with the vibrationally excited states. The frequencies and structures for all species of the \(\hbox {CN} + \hbox {H}_{2}/\hbox {D}_{2}\) reaction were calculated using G3 method for further kinetics calculation. The thermal rate constants were calculated using the conventional transition-state theory (TST) and canonical variational transition-state theory (CVT) by APUAMA code, over the temperature range from 200 to 4000 K. In addition, rate coefficients for vibrationally excited reactants CN (v = 1) or \(\hbox {H}_{2}\) (v = 1) or \(\hbox {D}_{2}\) (v = 1) are presented. The branching ratio for the partitioning into H/D + HCN/DCN or H/D + HNC/DNC has, also, been determined. The results showed that the \(\hbox {CN} (v=0) + \hbox {H}_{2} (v=0) \rightarrow \hbox {H} + \hbox {HCN} \) channel is dominant at all range of temperature, while \(\hbox {CN } (v=1) + \hbox {H}_{2} (v=0) \rightarrow \hbox {H} + \hbox {HNC}\) channel is dominant at T \(\ge \) 1900 K. The isotopic effects are the same behavior that \(\hbox {CN}(v=0,1) + \hbox {H}_{2}(v=0,1) \rightarrow \hbox {H} + \hbox {HCN/HNC}\) reactions. Reasonable agreement was found between the experimental results and the rate constants predicted by conventional transition-state theory, with tunneling correction, using the theoretical transition-state properties.







Similar content being viewed by others
References
Schacke H, Wagner HGg (1977) J Wolfrum Chem Phys 81:670
Sims IR, Smith IWM (1988) Chem Phys Lett 149:565
Sun Q, Yang DL, Bowman JM, Lin MC (1990) J Chem Phys 93:4730
He KG, Tokue I, Macdonald RG (1998) J Phys Chem A 102:4585. https://doi.org/10.1021/jp980875o
He KG, Tokue I, Harding LB, Macdonald RG (1998) J Phys Chem A 102:7653. https://doi.org/10.1021/jp982391y
Che DC, Liu K (1996) Chem Phys 207:367
Lai LH, Wang JH, Che DC, Liu K (1996) J Chem Phys 105:3332
Wang JH, Liu K, Schatz GC, ter Horst M (1997) J Chem Phys 107:7869
Pfeiffer JM, Metz RB, Thoemke JD, Woods E III, Crim FF (1996) J Chem Phys 104:4490
Kreher C, Theinl R, Gericke KH (1996) J Chem Phys 104:4481
Bair RA, Dunning TH (1985) J Chem Phys 82:2280
ter Horst MA, Schatz GC, Harding LB (1996) J Chem Phys 105:558
Carter S, Bowman JM, Harding LB (1997) Spectrochim Acta A 53:1179
Sun Q, Bowman JM (1990) J Chem Phys 92:5201
Bowman JM (1991) J Phys Chem 95:4960
Bowman JM, Schatz GC (1995) Annu Rev Phys Chem 46:169
Clary DC (1995) J Phys Chem 99:13664
Takayanagi T, Schatz GC (1997) J Chem Phys 106:3227
Bethardy GA, Wagner AF, Schatz GC, ter Host MA (1997) J Chem Phys 106:6001
Takayanagi T, Schatz GC (1997) Chem Phys Lett 265:410
Manthe U, Matzkies F (1998) Chem Phys Lett 282:442
Zhu W, Zhang JZH, Zhang YC, Zhang YB, Zhan LX, Zhang SL, Zhang DH (1998) J Chem Phys 108:3509
Correa E, e Silva WB, Barreto PRP, Albernaz AF (2017) J Mol Model 23:169
Zhao R, Gao D, Pan X, Song L, Yu H, Yu S, Yao L (2019) Chem Phys 516:38
Kaledin AL, Haeven MC, Bowman JM (1999) J Chem Phys 100:10380
Euclides HO, Barreto PRP (2017) J Mol Model 23:176
Curtiss LA, Raghavachari K, Redfern PC, Rassolov V, Pople JA (1998) J Chem Phys 109:7764
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) Chem Phys Lett 157:479
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2009) Gaussian 09. Gaussian Inc, Wallingford
Fernández-Ramos A, Miller JA, Klippenstein SJ, Truhlar DG (2006) Chem Rev 106:4518
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure. IV. Constants of diatomic molecules. Van Nostrand Reinhold Co., New York
Irikura KK (2007) J Phys Chem Ref Data 36(2):389
Wagner AF, Bair RA (1986) Int Chem Kinet 18:473
Kim K, Kin WT (1979) J Chem Phys 71:1967
da Silva WB, Albernaz AF, Barreto PRP, Correa E (2017) J Mol Model 23:143
da Silva WB, Gargano R, e Silva GM, Albernaz AF (2016) Rev Virt Quim 8:515
Balucani N, Leonori F, Petrucci R, Wang X, Casavecchia P, Skouteris D, Albernaz AF, Gargano R (2015) Chem Phys 449:34
Gurvich LV, Veyts IV, Alcock CB (1989) Thermodynamic properties of individual substances, Fouth edn. Hemisphere Pub. Co., New York
Murrell JN, Farantos SC, Huxley P, Varandas AJC (1984) Molecular potential energy functions. Oxford University Press, New York
Dunham JL (1932) Phys Rev 41:713
Hammond G (1955) J Am Chem Soc 77:334
Atakan B, Jacobs A, Wahl M, Weller R (1989) J Wolfrum Chem Phys Lett 154:449
Balla RJ, Pasternack L (1987) J Phys Chem 91:73
Wang X, Bowman M (2013) J Chem Theory Comput 9:901
Jiang B, Guo H (2013) J Chem Phys 139:224310
Sumathi R, Nguyen MT (1998) J Phys Chem A 102:8013
Ju L-P, Han K-L, Zang JZH (2006) J Theory Comput Chem 4:769
Johnston GW, Bersohn R (1989) J Chem Phys 90:7096
Jacobs A, Wahl M, Weller R (1989) J Wolfrum Symp Int Combust Proc 22:1093
Choi N, Blitz MA, McKee K, Pilling MJ, Seakins PW (2004) Chem Phys Lett 68:384
Natarajan K, Roth P (1988) Symp Int Combust Proc 21:729
Wooldridge ST, Hanson RK, Bowman CT (1996) Int J Chem Phys Kinet 28:245
Baulch DL, Cobos CJ, Cox RA, Frank P, Hayman G, Just Th, Kerr JA, Murrells T, Pilling MJ, Troe J, Walker RW, Warnatz J (1994) J Phys Chem Ref Data 23:847
Tsang W (1992) J Phys Chem Ref Data 21:753
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albernaz, A.F., Barreto, P.R.P. Theoretical studies of \({{\mathrm{{CN} + {H}}_{2}({\mathrm{D}}_{2})}}\) reactions: competition between H(D)-abstractions in \({\mathrm{H(D) + HCN(DCN)/HNC(DNC)}} \) channels. Theor Chem Acc 138, 93 (2019). https://doi.org/10.1007/s00214-019-2479-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2479-1