Abstract
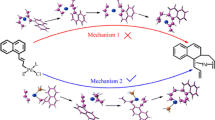
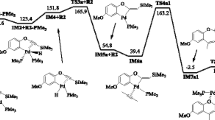
Density functional theory (DFT) calculations have been performed on a homogeneous gold-catalyzed reaction between acetylene/propyne and the cyclic ketal 2,2-dimethyl-1,3-dioxolane, DMDO, in the presence of water, with the aim of understanding the actual role played by water. After the addition of DMDO to the alkyne, hydrolysis may happen through two possible reaction routes. In the so-called H-route (previously proposed for similar intramolecular reactions), a water proton is initially added to the alkyne C atom still linked to gold and, afterwards, an OH group adds to a DMDO C atom to allow the release of acetone, whereas in the newly proposed OH-route, a water OH group firstly adds to the most substituted DMDO C atom with simultaneous addition of H to the alkyne C atom linked to gold. A 1,3-H transposition from the just added OH group allows the release of acetone. An intramolecular nucleophilic OH addition to the gold-activated alkene intermediate formed from both hydrolysis paths drives the system to the corresponding 1,3-dioxolane product. The H-route is unable to explain the formation of the dioxolane addition products (observed in similar intramolecular reactions) instead of those coming from the direct addition of water to the alkyne, since it is energetically more demanding than the direct hydration. However, OH-route goes through structures that are more stable than those in the water addition, so, it is the one actually happening for the reaction under study. The regioselective addition of DMDO to the internal C atom of propyne is predicted on the basis of the large polarity of the structures formed in this approach, which makes them capable of strong interactions with water.









Similar content being viewed by others
References
Hashmi ASK (2007) Gold-catalyzed organic reactions. Chem Rev 107:3180–3211
Dorel R, Echavarren AM (2015) Gold(I)-catalyzed activation of alkynes for the construction of molecular complexity. Chem Rev 115:9028–9072
Teles JH, Brode S, Chabanas M (1998) Cationic gold(I) complexes: highly efficient catalysts for the addition of alcohols to alkynes. Angew Chemie Int Ed 37:1415–1418
Corma A, Leyva-Pérez A, Sabater MJ (2011) Gold-catalyzed carbon–heteroatom bond-forming reactions. Chem Rev 111:1657–1712
Zi W, Dean Toste F (2016) Recent advances in enantioselective gold catalysis. Chem Soc Rev 45:4567–4589
Belting V, Krause N (2006) Gold-catalyzed tandem cycloisomerization-hydroalkoxylation of homopropargylic alcohols. Org Lett 8:4489–4492
Hashmi ASK, Bührle M, Wölfle M, Rudolph M, Wieteck M, Rominger F, Frey W (2010) Gold catalysis: tandem reactions of diyne-diols and external nucleophiles as an easy access to tricyclic cage-like structures. Chem Eur J 16:9846–9854
Ramón RS, Pottier C, Gómez-Suárez A, Nolan SP (2011) Gold(I)-catalyzed tandem alkoxylation/lactonization of γ-hydroxy-α, β-acetylenic esters. Adv Synth Catal 353:1575–1583
Panda B, Sarkar TK (2013) Gold catalysis: regio- and stereoselective total synthesis of xyloketals D and G and the related natural product alboatrin. J Org Chem 78:2413–2421
Alcaide B, Almendros P, Carrascosa R (2012) Gold-catalyzed direct cycloketalization of acetonide-tethered alkynes in the presence of water. Tetrahedron 68:9391–9396
Alcaide B, Almendros P, Carrascosa R, López R, Menéndez MI (2012) Gold-catalyzed bis-cyclization of 1,2-diol-or acetonide-tethered alkynes. Synthesis of β-lactam-bridged acetals: a combined experimental and theoretical study. Tetrahedron 68:10748–10760
Alcaide B, Almendros P, Martínez del Campo T, Torres MR (2013) Synthesis of fused-β-lactams through selective gold-catalyzed oxycyclization of dioxolane-tethered enynes. J Org Chem 78:8956–8965
Aepkers M, Wünsch B (2005) Structure-affinity relationship studies of non-competitive NMDA receptor antagonists derived from dexoxadrol and etoxadrol. Bioorg Med Chem 13:6836–6849
Wang W, Hammond GB, Xu B (2012) Ligand effects and ligand design in homogeneous gold(I) catalysis. J Am Chem Soc 134:5697–5705
Biasiolo L, Trinchillo M, Belanzoni P, Belpassi L, Busico V, Ciancaleoni G, D’Amora A, Macchioni A, Tarantelli F, Zuccaccia D (2014) Unexpected Anion effect in the alkoxylation of alkynes catalyzed by N-heterocyclic carbene (NHC) cationic gold complexes. Chem A Eur J 20:14594–14598
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09. B.01 edn. Gaussian Inc., Wallingford CT
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3:214–218
Li X (2006) Energy-represented direct inversion in the iterative subspace within a hybrid geometry optimization method. J Chem Theory Comput 2:835–839
Barone V, Cossi M, Tomasi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem 19:404–417
Tomasi J, Mennucci B, Cancès E (1999) The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level. J Mol Struct THEOCHEM 464:211–226
Scalmani G, Frisch MJ (2010) Continuous surface charge polarizable continuum models of solvation. I. General formalism. J Chem Phys 132:114110/1–114110/15
Rappe AK, Casewit CJ, Colwell KS, Goddard WA III, Skiff WM (1992) UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114:10024–10039
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Dunning TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1013
Kendall RA, Dunning TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796–6806
Peterson KA, Figgen D, Dolg M, Stoll H (2007) Energy-consistent relativistic pseudopotentials and correlation consistent basis sets for the 4d elements Y–Pd. J Chem Phys 126:124101/1–124101/12
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Kovács Gábor, Ujaque Gregori, Lledós A (2008) The reaction mechanism of the hydroamination of alkenes catalyzed by gold(I)–phosphine: the role of the counterion and the N-nucleophile substituents in the proton-transfer step. J Am Chem Soc 130:853–864
Glendening ED, Reed AE, Carpenter JE, Weinhold F. (2012) NBO Version 3.1, University of Wisconsin, Madison
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor–acceptor persective. Cambridge University Press, Cambridge
Pyykkö P (2004) Theoretical chemistry of gold. Angew Chemie Int Ed 43:4412–4456
Nieto-Faza O, Álvarez-Rodríguez R, Silva-López C (2011) Performance of density functional theory on homogeneous gold catalysis. Theor Chim Acta 128:647–661
Alcaide B, Almendros P, Quirós MT, López R, Menéndez MI, Sochacka-Ćwikła A (2013) Unveiling the reactivity of propargylic hydroperoxides under gold catalysis. J Am Chem Soc 135:898–905
Vergara E, Cerrada E, Casini A, Zava O, Laguna M, Dyson PJ (2010) Antiproliferative activity of gold(I) alkyne complexes containing water-soluble phosphane ligands. Organometallics 29:2596–2603
Hashmi ASK (2014) Dual gold catalysis. Acc Chem Res 47:864–876
Mazzone G, Russo N, Sicilia E (2015) Catalytic role of dinuclear σ,π-acetylide gold(I) complexes in the hydroamination of terminal alkynes: theoretical insights. J Chem Theory Comput 11:581–590
Hassanali A, Giberti F, Cuny J, Kühne TD, Parrinello M (2013) Proton transfer through the water gossamer. Proc Natl Acad Sci USA 110:13723–13728
Mazzole G, Russo N, Sicilia E (2010) Gold(I)-catalyzed hydration of 1,2-diphenylacetylene: computational insights. J Chem Theory Comput 6:2782–2789
Mazzole G, Russo N, Sicilia E (2012) Homogeneous gold catalysis; Hydration of 1,2-diphenylacetylene with methanol in aqueous media. A theoretical viewpoint. Organometallics 31:3074–3080
Krauter CM, Hashmi ASK, Pernpointner M (2010) A new insight into gold(I)-catalyzed hydration of alkynes: proton transfer. ChemCatChem 2:1226–1230
Acknowledgements
The authors thank Ministerio de Economía y Competitividad of Spain (Grant No. CTQ2015-70231-P) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as part of the special collection of articles derived from the 11th Congress on Electronic Structure: Principles and Applications (ESPA-2018).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernández-Pulido, Y., López, R. & Menéndez, M.I. Gold(I)-catalyzed intermolecular dioxolane addition to alkynes: the role of water. Theor Chem Acc 138, 74 (2019). https://doi.org/10.1007/s00214-019-2460-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2460-z