Abstract
Rationale
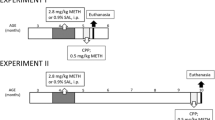
Abuse of the psychostimulant methamphetamine (METH) can cause long-lasting damage to brain monoaminergic systems and is associated with profound mental health problems for users, including lasting cognitive impairments. Animal models of METH exposure have been useful in dissecting the molecular effects of the drug on cognition, but many studies use acute, non-contingent “binge” administrations of METH which do not adequately approximate human METH use. Long-term METH exposure via long-access (LgA) self-administration paradigms has been proposed to more closely reflect human use and induce cognitive impairments.
Objective
To better understand the role of contingency and patterns of exposure in METH-induced cognitive impairments, we analyzed behavioral and neurochemical outcomes in adult male rats, comparing non-contingent “binge” METH administration with contingent (LgA) METH self-administration and non-contingent yoked partners.
Results
Binge METH (40 mg/kg, i.p., over 1 day) dramatically altered striatal and hippocampal dopamine, DOPAC, 5-HT, 5-HIAA, BDNF, and TrkB 75 days after drug exposure. In contrast, 6-h LgA METH self-administration (cumulative 24.8–48.9 mg METH, i.v., over 16 days) altered hippocampal BDNF in both contingent and yoked animals but reduced striatal 5-HIAA in only contingent animals. Neurochemical alterations following binge METH administration were not accompanied by cognitive deficits in Morris water maze, novel object recognition, or Y-maze tests. However, contingent LgA METH self-administration resulted in impaired spatial memory in the water maze.
Conclusions
Overall, substantial differences in neurochemical markers between METH exposure and self-administration paradigms did not consistently translate to deficits in cognitive tasks, highlighting the complexity of correlating METH-induced neurochemical changes with cognitive outcomes.









Similar content being viewed by others
References
Anderson EM, Wissman AM, Chemplanikal J, Buzin N, Guzman D, Larson EB, Neve RL, Nestler EJ, Cowan CW, Self DW (2017) BDNF-TrkB controls cocaine-induced dendritic spines in rodent nucleus accumbens dissociated from increases in addictive behaviors. Proc Natl Acad Sci U S A 114:9469–9474
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Belcher AM, O’Dell SJ, Marshall JF (2005) Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology 30:2026–2034
Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1:1306–1311
Bisagno V, Ferguson D, Luine VN (2002) Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res 940:95–101
Braun AA, Herring NR, Schaefer TL, Hemmerle AM, Dickerson JW, Seroogy KB, Vorhees CV, Williams MT (2011) Neurotoxic (+)-methamphetamine treatment in rats increases brain-derived neurotrophic factor and tropomyosin receptor kinase B expression in multiple brain regions. Neuroscience 184:164–171
Cadet JL, Bisagno V (2015) Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry 6:189
Camarasa J, Rodrigo T, Pubill D, Escubedo E (2010) Memantine is a useful drug to prevent the spatial and non-spatial memory deficits induced by methamphetamine in rats. Pharmacol Res 62:450–456
Chapman DE, Hanson GR, Kesner RP, Keefe KA (2001) Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther 296:520–527
Cornett EM, Goeders NE (2013) 96-hour methamphetamine self-administration in male and female rats: a novel model of human methamphetamine addiction. Pharmacol Biochem Behav 111:51–57
Cox BM, Cope ZA, Parsegian A, Floresco SB, Aston-Jones G, See RE (2016) Chronic methamphetamine self-administration alters cognitive flexibility in male rats. Psychopharmacology 233:2319–2327
Daberkow DP, Kesner RP, Keefe KA (2005) Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav 81:198–204
Dean AC, Groman SM, Morales AM, London ED (2013) An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38:259–274
Dworkin SI, Co C, Smith JE (1995) Rat brain neurotransmitter turnover rates altered during withdrawal from chronic cocaine administration. Brain Res 682:116–126
Edwards S, Koob GF (2013) Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol 24:356–362
Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR (2011) Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. J Pharmacol Exp Ther 336:809–815
Friedman SD, Castaneda E, Hodge GK (1998) Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav 61:35–44
Gould TJ (2010) Addiction and cognition. Addict Sci Clin Pract 5:4–14
Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P (2001) BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411:86–89
Gutierrez A, Jablonski SA, Amos-Kroohs RM, Barnes AC, Williams MT, Vorhees CV (2017) Effects of housing on methamphetamine-induced neurotoxicity and spatial learning and memory. ACS Chem Neurosci 8:1479–1489
Hart CL, Marvin CB, Silver R, Smith EE (2012) Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology 37:586–608
He J, Yang Y, Yu Y, Li X, Li XM (2006) The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav Brain Res 172:39–45
Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT (2008) Effect of +−methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology 199:637–650
Hilburn C, Nejtek VA, Underwood WA, Singh M, Patel G, Gangwani P, Forster MJ (2011) Is serum brain-derived neurotrophic factor related to craving for or use of alcohol, cocaine, or methamphetamine? Neuropsychiatr Dis Treat 7:357–364
Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN (2003) Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci 24:566–573
Jang CG, Whitfield T, Schulteis G, Koob GF, Wee S (2013) A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology 225:753–763
Johansen A, McFadden LM (2017) The neurochemical consequences of methamphetamine self-administration in male and female rats. Drug Alcohol Depend 178:70–74
Kalechstein AD, Newton TF, Green M (2003) Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatr Clin Neurosci 15:215–220
Keck TM, Yang HJ, Bi GH, Huang Y, Zhang HY, Srivastava R, Gardner EL, Newman AH, Xi ZX (2013) Fenobam sulfate inhibits cocaine-taking and cocaine-seeking behavior in rats: implications for addiction treatment in humans. Psychopharmacology 229:253–265
Keck TM, Zou MF, Bi GH, Zhang HY, Wang XF, Yang HJ, Srivastava R, Gardner EL, Xi ZX, Newman AH (2014) A novel mGluR5 antagonist, MFZ 10-7, inhibits cocaine-taking and cocaine-seeking behavior in rats. Addict Biol 19:195–209
Kish SJ, Boileau I, Callaghan RC, Tong J (2017) Brain dopamine neurone ‘damage’: methamphetamine users vs. Parkinson’s disease - a critical assessment of the evidence. Eur J Neurosci 45:58–66
Kobeissy FH, Mitzelfelt JD, Fishman I, Morgan D, Gaskins R, Zhang Z, Gold MS, Wang KK (2012) Methods in drug abuse models: comparison of different models of methamphetamine paradigms. Methods Mol Biol 829:269–278
Kousik SM, Carvey PM, Napier TC (2014) Methamphetamine self-administration results in persistent dopaminergic pathology: implications for Parkinson’s disease risk and reward-seeking. Eur J Neurosci 40:2707–2714
Krasnova IN, Ladenheim B, Jayanthi S, Oyler J, Moran TH, Huestis MA, Cadet JL (2001) Amphetamine-induced toxicity in dopamine terminals in CD-1 and C57BL/6J mice: complex roles for oxygen-based species and temperature regulation. Neuroscience 107:265–274
Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL (2010) Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One 5:e8790
Krasnova IN, Chiflikyan M, Justinova Z, McCoy MT, Ladenheim B, Jayanthi S, Quintero C, Brannock C, Barnes C, Adair JE, Lehrmann E, Kobeissy FH, Gold MS, Becker KG, Goldberg SR, Cadet JL (2013) CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol Dis 58:132–143
Kuczenski R, Segal DS, Melega WP, Lacan G, McCunney SJ (2009) Human methamphetamine pharmacokinetics simulated in the rat: behavioral and neurochemical effects of a 72-h binge. Neuropsychopharmacology 34:2430–2441
Li X, Wolf ME (2015) Multiple faces of BDNF in cocaine addiction. Behav Brain Res 279:240–254
Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Ford KA, Ferrario CR, Loweth JA, Wolf ME (2013) Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci 33:1130–1142
Marshall JF, Belcher AM, Feinstein EM, O’Dell SJ (2007) Methamphetamine-induced neural and cognitive changes in rodents. Addiction 102(Suppl 1):61–69
Mathiasen JR, DiCamillo A (2010) Novel object recognition in the rat: a facile assay for cognitive function. Curr Protoc Pharmacol 49:5.59.1–5.59.15. https://doi.org/10.1002/0471141755.ph0559s49
McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenyak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE (2012) Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther 340:295–303
McGinty JF, Whitfield TW Jr, Berglind WJ (2010) Brain-derived neurotrophic factor and cocaine addiction. Brain Res 1314:183–193
Melo P, Magalhaes A, Alves CJ, Tavares MA, de Sousa L, Summavielle T, Moradas-Ferreira P (2012) Methamphetamine mimics the neurochemical profile of aging in rats and impairs recognition memory. Neurotoxicology 33:491–499
Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ (2005) Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry 13:141–154
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
North A, Swant J, Salvatore MF, Gamble-George J, Prins P, Butler B, Mittal MK, Heltsley R, Clark JT, Khoshbouei H (2013) Chronic methamphetamine exposure produces a delayed, long-lasting memory deficit. Synapse 67:245–257
Parsegian A, Glen WB Jr, Lavin A, See RE (2011) Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry 69:253–259
Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD (2012) Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology 37:1275–1287
Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2011) Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36:782–792
Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE (2012) Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology 62:1119–1126
Ren Q, Ma M, Yang C, Zhang JC, Yao W, Hashimoto K (2015) BDNF-TrkB signaling in the nucleus accumbens shell of mice has key role in methamphetamine withdrawal symptoms. Transl Psychiatry 5:e666
Ren W, Tao J, Wei Y, Su H, Zhang J, Xie Y, Guo J, Zhang X, Zhang H, He J (2016) Time-dependent serum brain-derived neurotrophic factor decline during methamphetamine withdrawal. Medicine (Baltimore) 95:e2604
Ren W, Luan X, Zhang J, Gutteea P, Cai Y, Zhao J, Gu Y, Wu C, Su H, Tao J, Xie Y, Lv D, Feng L, He J (2017) Brain-derived neurotrophic factor levels and depression during methamphetamine withdrawal. J Affect Disord 221:165–171
Richards JB, Sabol KE, de Wit H (1999) Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology 146:432–439
Rogers JL, De Santis S, See RE (2008) Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology 199:615–624
Rusyniak DE (2013) Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin N Am 36:261–275
Schroder N, O’Dell SJ, Marshall JF (2003) Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse 49:89–96
Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW (2009) Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther 331:555–562
Seiden LS, Fischman MW, Schuster CR (1976) Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend 1:215–219
Seiden LS, Woolverton WL, Lorens SA, Williams JE, Corwin RL, Hata N, Olimski M (1993) Behavioral consequences of partial monoamine depletion in the CNS after methamphetamine-like drugs: the conflict between pharmacology and toxicology. NIDA Res Monogr 136:34–46 discussion 46-52
Seyedhosseini Tamijani SM, Beirami E, Ahmadiani A, Dargahi L (2018) Effect of three different regimens of repeated methamphetamine on rats’ cognitive performance. Cogn Process 19:107–115
Shabani S, Houlton SK, Hellmuth L, Mojica E, Mootz JR, Zhu Z, Reed C, Phillips TJ (2016) A mouse model for binge-level methamphetamine use. Front Neurosci 10:493
Simoes PF, Silva AP, Pereira FC, Marques E, Grade S, Milhazes N, Borges F, Ribeiro CF, Macedo TR (2007) Methamphetamine induces alterations on hippocampal NMDA and AMPA receptor subunit levels and impairs spatial working memory. Neuroscience 150:433–441
Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR (1999) Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol 371:123–135
Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP (2004) Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology 175:68–75
Tunstall BJ, Ho CP, Cao J, Vendruscolo JCM, Schmeichel BE, Slack RD, Tanda G, Gadiano AJ, Rais R, Slusher BS, Koob GF, Newman AH, Vendruscolo LF (2018) Atypical dopamine transporter inhibitors attenuate compulsive-like methamphetamine self-administration in rats. Neuropharmacology 131:96–103
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Wagner GC, Seiden LS, Schuster CR (1979) Methamphetamine-induced changes in brain catecholamines in rats and Guinea pigs. Drug Alcohol Depend 4:435–438
Wenk GL (2004) Assessment of spatial memory using the radial arm maze and Morris water maze. Curr Protoc Neurosci 26:8.5A.1–8.5A.12. https://doi.org/10.1002/0471142301.ns0805as26
Wiskerke J, Schoffelmeer AN, De Vries TJ (2016) Response contingency directs long-term cocaine-induced neuroplasticity in prefrontal and striatal dopamine terminals. Eur Neuropsychopharmacol 26:1667–1672
Wood S, Sage JR, Shuman T, Anagnostaras SG (2014) Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev 66:193–221
Xi ZX, Kleitz HK, Deng X, Ladenheim B, Peng XQ, Li X, Gardner EL, Stein EA, Cadet JL (2009) A single high dose of methamphetamine increases cocaine self-administration by depletion of striatal dopamine in rats. Neuroscience 161:392–402
Funding
The authors received support from the NIDA-IRP and the NIA-IRP for this research. TMK was supported by an IRTA postdoctoral fellowship. CAS and CB were supported by IRTA post-baccalaureate fellowships. AHN is supported by Z1A DA000424.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (US National Academy of Sciences) and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse under protocols 09-CNRB-25 and 13-BNRB-48.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schweppe, C.A., Burzynski, C., Jayanthi, S. et al. Neurochemical and behavioral comparisons of contingent and non-contingent methamphetamine exposure following binge or yoked long-access self-administration paradigms. Psychopharmacology 237, 1989–2005 (2020). https://doi.org/10.1007/s00213-020-05513-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05513-z