Abstract
Rationale
Posttraumatic stress disorder (PTSD) and alcoholism are frequently comorbid, suggesting the possibility of overlapping neural substrates. The neurokinin 1 (NK1) receptor for substance P (SP) has been implicated in both stress- and alcohol-related behaviors. The NK1 antagonist aprepitant, clinically available as a treatment for chemotherapy-induced nausea, offers a tool to probe a potential role of the SP/NK1 system in comorbid PTSD and alcoholism.
Objectives
The aim of this study is to evaluate the efficacy of aprepitant for treatment of comorbid PTSD and alcoholism.
Methods
Fifty-three patients with PTSD and alcoholism were admitted for 4 weeks to an inpatient unit at the NIH Clinical Center and randomized to double-blind aprepitant (125 mg/day; based on PET studies reporting >90 % central receptor occupancy at this dose) or placebo. After reaching steady state, subjects were assessed for PTSD symptom severity, behavioral and neuroendocrine responses to stress and alcohol cues, and functional magnetic resonance imaging (fMRI) responses to stimuli with positive or negative emotional valence.
Results
Aprepitant treatment had no effect on PTSD symptoms or subjective or physiological responses to stress or alcohol cues. However, aprepitant robustly potentiated ventromedial prefrontal cortex (mPFC) fMRI responses to aversive visual stimuli.
Conclusions
Despite the lack of effect on PTSD symptoms and responses to stress/alcohol cues, NK1 antagonism activated the ventral mPFC, an area considered hypoactive in PTSD, during exposure to aversive stimuli. Because this brain area is critically important for extinction of fear memories and in alcohol craving and relapse, our finding suggests that NK1 antagonism might be a useful pharmacological treatment to enhance extinction-based cue-exposure therapies.



Similar content being viewed by others
References
Acheson DT, Gresack JE, Risbrough VB (2012) Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62:674–685
Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S (2005) Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res 29:1351–1355
Bergstrom M, Hargreaves RJ, Burns HD et al (2004) Human positron emission tomography studies of brain neurokinin 1 receptor occupancy by aprepitant. Biol Psychiatry 55:1007–1012
Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J (1994) Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatr 151:1132–1136
Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–606
Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013) The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl)
Brady KT, Waldrop AE, McRae AL, Back SE, Saladin ME, Upadhyaya HP, Anton RF, Randall PK (2006) The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. J Stud Alcohol 67:700–706
Coffey SF, Stasiewicz PR, Hughes PM, Brimo ML (2006) Trauma-focused imaginal exposure for individuals with comorbid posttraumatic stress disorder and alcohol dependence: revealing mechanisms of alcohol craving in a cue reactivity paradigm. Psychol Addict Behav 20:425–435
Coffey SF, Schumacher JA, Stasiewicz PR, Henslee AM, Baillie LE, Landy N (2010) Craving and physiological reactivity to trauma and alcohol cues in posttraumatic stress disorder and alcohol dependence. Exp Clin Psychopharmacol 18:340–349
Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L (1997) Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol 106:243–250
Costa PT, McCrae RR (2002) NEO Personality Inventory-Revised (NEO PI-R). APA, Washington, DC
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173
de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG (2006) Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res 40:550–567
First MB, Spitzer RL, Gibbon M, Williams JBW (1995) Structured clinical interview for DSM-IV patient edition. Biometric Research, New York
Foa EB, Riggs DS, Dancu CV, Rothbaum BO (1993) Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress 6:459–473
Fox HC, Bergquist KL, K-I H, Sinha R (2007) Stress-induced and alcohol cue-induced craving in recently-abstinent alcohol dependent individuals. Alcohol Clin Exp Res 31:395–403
Frank R, Hargreaves R (2003) Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov 2:566–580
George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M (2008) Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 319:1536–1539
Geracioti TD Jr, Carpenter LL, Owens MJ, Baker DG, Ekhator NN, Horn PS, Strawn JR, Sanacora G, Kinkead B, Price LH, Nemeroff CB (2006) Elevated cerebrospinal fluid substance p concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry 163:637–643
Gillespie CF, Phifer J, Bradley B, Ressler KJ (2009) Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety 26:984–992
Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ (2011) Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology (Berl) 218:121–129
Institute of Medicine. Committee on Treatment of Posttraumatic Stress Disorder (2008) Treatment of posttraumatic stress disorder: an assessment of the evidence. National Academies Press, Washington, DC
Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, Hargreaves R, Hietala J, Lines C, Beebe K, Reines S (2006) Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biol Psychiatry 59:216–223
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52:1048–1060
Kirschbaum C, Pirke KM, Hellhammer DH (1993) The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
Kramer MS et al (1998) Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 281:1640–1645
Kwako LE, Schwandt ML, Sells JR, Ramchandani VA, Hommer DW, George DT, Sinha R, Heilig, M (2014) Methods for inducing alcohol craving in individuals with comorbid alcohol dependence and posttraumatic stress disorder: behavioral and physiological outcomes. Addict Biol (e-publication ahead of print)
Lang PJ, Bradley MM, Cuthbert BN (1999) International affective picture system (IAPS): technical manual and affective ratings. In: The Center for Research in Psychophysiology, University of Florida, Gainesville, FL
Liberzon I, Sripada CS (2008) The functional neuroanatomy of PTSD: a critical review. Prog Brain Res 167:151–169
Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA (2014) Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci 17:106–113
Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ (2000) Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res 24:651–658
Matsumoto D, Ekman P (1988) Japanese and Caucasian facial expressions of emotion and neutral faces (JACFEE and JACNeuF). Human Interaction Laboratory, University of California, San Francisco, 401
McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA (2006) Stress reactivity: biological and subjective responses to the cold pressor and Trier Social stressors. Hum Psychopharmacol 21:377–385
Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007) Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62:446–454
Mills KL, Teeson M, Back SE, Brady KT, Baker AL, Hopwood S, Sannibale C, Barrett EL, Merz S, Rosenfeld J, Ewer PL (2012) Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: a randomized controlled trial. JAMA 308:690–699
Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR (1987) Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol 96:122–126
Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y (2009) Long-lasting incubation of conditioned fear in rats. Biol Psychiatry 65:881–886
Quirk GJ, Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33:56–72
Ratti E, Bellew K, Bettica P, Bryson H, Zamuner S, Archer G, Squassante L, Bye A, Trist D, Krishnan KR, Fernandes S (2011) Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. J Clin Psychopharmacol 31:727–733
Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, Wunschel SM, Abrams DB (1994) Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol 62:620–626
Santa Ana EJ, Saladin ME, Back SE, Waldrop AE, Spratt EG, McRae AL, LaRowe SD, Timmerman MA, Upadhyaya H, Brady KT (2006) PTSD and the HPA axis: differences in response to the cold pressor task among individuals with child vs. adult trauma. Psychoneuroendocrinology 31:501–509
Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M (2011) Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 218:111–119
Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M (2012) Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron 76:192–208
Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R (2013) Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry 70:727–739
Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK (2004) Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 61:168–176
Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL (2005) A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 62:273–281
Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM (2009) Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34:1198–1208
Sinha R, Shaham Y, Heilig M (2011a) Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 218:69–82
Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ (2011b) Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry 68:942–952
Skinner HA (1984) Assessing alcohol-use by patients in treatment. Res Adv Alcohol Drug 8:183–207
Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E (1986) The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav 11:149–161
Spielberger CD, Gorsuch RL, Lushene RE (1970) The state-trait anxiety inventory. Consulting Psychologists Press, Palo Alto
Stasiewicz PR, Gulliver SB, Bradizza CM, Rohsenow DJ, Torrisi R, Monti PM (1997) Exposure to negative emotional cues and alcohol cue reactivity with alcoholics: a preliminary investigation. Behav Res Ther 35:1143–1149
Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF (2012) Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32:7563–7571
Weathers FW, Keane TM, Davidson JR (2001) Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 13:132–156
Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S (2006) Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry 59:582–587
Zamuner S, Rabiner EA, Fernandes SA, Bani M, Gunn RN, Gomeni R, Ratti E, Cunningham VJ (2012) A pharmacokinetic PET study of NK(1) receptor occupancy. Eur J Nucl Med Mol Imaging 39:226–235
Acknowledgments
This work was carried out under a Clinical Trial Agreement (CTA) between the US Government and Merck & Company, Incorporated. Merck had no role in study design, collection, analysis or interpretation of the data, or drafting of the manuscript. We thank the following individuals and departments: Lauren Adams, Joanna Sells, Jessica Berman, Eric Markey, Victoria Brown, Keva Garg, and Byung Joon Park; Debra Hill, Cheryl Jones, and Monte Phillips; and James Paterson, Judie Jones, Mary Ley, Jacqueline Goodson, and the 1SE nursing staff of the NIH Clinical Center.
Conflict of interest
Drs. Kwako, George, Schwandt, Spagnolo, Momenan, Hommer, Shaham, and Heilig, as well as Ms. Diamond, declare no competing financial interests. Dr. Sinha is a member of the Embera Neurotherapeutics Scientific Advisory Board. This research was supported by the Division of Intramural Clinical and Biological Research, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Laura E. Kwako and David T. George contributed equally to this work.
Daniel W. Hommer—deceased.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
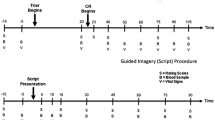
Timeline of challenge procedures. Graphical representation of Trier/CR and Script procedures. The numbers represent minutes, i.e., in relation to the initiation of the procedure. S = scales, B = blood samples, V = vital signs. (GIF 26 kb)
Supplementary Figure 2
Cortisol response to challenge procedures. Due to missing data for cortisol levels, the sample sizes are reduced for these analyses. A) Effect of script type on cortisol levels. Covariates in the model included gender, ADS score, and total score from the ASI. B) Effect of aprepitant treatment on cortisol levels in response to the alcohol cue script. Covariates in the model included gender, ADS score, and total score from the ASI. C) Effect of aprepitant treatment on cortisol levels in response to the stress script. Covariates in the model included gender, ADS score, and total score from the ASI. D) Effect of aprepitant treatment on cortisol levels in response to the Trier/CR. Covariates in the model included gender, race, ADS score, and neuroticism. (GIF 87 kb)
Supplementary Figure 3
ACTH response to challenge procedures. Due to missing data for ACTH levels, the sample sizes are reduced for these analyses. A) Effect of script type on ACTH levels. Covariates in the model included gender, number of heavy drink days, total score from the CTQ, and total score from the ASI. B) Effect of aprepitant treatment on ACTH levels in response to the alcohol cue script. Covariates in the model included gender, age, overall PSSI severity score, CTQ total score, and total score from the ASI. C) Effect of aprepitant treatment on ACTH levels in response to the stress script. Covariates in the model included gender. D) Effect of aprepitant treatment on ACTH levels in response to the Trier/CR. Covariates in the model included gender and number of heavy drink days. (GIF 101 kb)
Supplementary Table 1
Reported side effects. This table includes side effects reported by participants, organized by group. Data are reported for all subjects for 10 side effects, and eight additional side effects are reported for a subset of participants. These side effects were added to those collected part-way through the study, which accounts for the reduced sample size. (DOCX 60 kb)
Rights and permissions
About this article
Cite this article
Kwako, L.E., George, D.T., Schwandt, M.L. et al. The neurokinin-1 receptor antagonist aprepitant in co-morbid alcohol dependence and posttraumatic stress disorder: a human experimental study. Psychopharmacology 232, 295–304 (2015). https://doi.org/10.1007/s00213-014-3665-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3665-4