Abstract
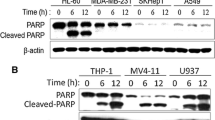
Coenzyme Q (CoQ) analogs with variable numbers of isoprenoid units have been demonstrated as anticancer and antioxidant/pro-oxidant molecules. This study examined the in vitro and in vivo antitumor and apoptosis activities of CoQ0 (2,3-dimethoxy-5-methyl-1,4-benzoquinone, zero isoprenoid side-chains) through upregulation of the Voltage‐dependent anion channel 1 (VDAC1) signaling pathway on human promyelocytic leukemia. CoQ0 (0–40 μg/mL) treatment significantly reduced HL-60 cell viability, and up-regulated mitochondrial VDAC1 expression. CoQ0 treatment triggers intracellular ROS generation, calcium release, ΔΨm collapse and PTP opening in HL-60 cells. CoQ0 treatment induced apoptosis, which was associated with DNA fragmentation, cytochrome c release, caspase-3 and PARP activation, and Bax/Bcl-2 dysregulation. Annexin V-PI staining indicated that CoQ0 promotes late apoptosis. Furthermore, the blockade of CoQ0-induced ROS production by antioxidant NAC pretreatment substantially attenuated CoQ0-induced apoptosis. The activation of p-GSK3β expression, cyclophilin D inhibition, and p53 activation through ROS are involved in CoQ0-induced HL-60 apoptotic cell death. Notably, ROS-independent p38 activation is involved in CoQ0-mediated apoptosis in HL-60 cells. In addition, the silencing of VDAC1 also prevented CoQ0-induced mitochondrial translocation of Bax, activation of caspase-3, and reduction in Bcl-2. Intriguingly, VDAC1 silencing did not prevent ROS production induced by CoQ0, which in turn indicates that CoQ0 induced ROS-mediated VDAC1 and then mitochondrial apoptosis in HL-60 cells. In vivo results revealed that CoQ0 is effective in delaying tumor incidence and reducing the tumor burden in HL-60-xenografted nude mice. Taken together, CoQ0 could be a promising anticancer agent for the treatment of human promyelocytic leukemia through upregulation of VDAC1 signaling pathways.












Similar content being viewed by others
Abbreviations
- CoQ0 :
-
Coenzyme Q0
- VDAC1:
-
Voltage‐dependent anion channel 1
- ΔΨ:
-
Mitochondrial membrane potential
- PTP:
-
Permeability transition pore
- ROS:
-
Reactive oxygen species
- NAC:
-
N-acetylcysteine
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- zVAD-FMK:
-
Z-Val-Ala-Asp-fluoromethylketone
- PI:
-
Propidium iodide
References
Armstrong JS, Whiteman M, Rose P, Jones DP (2003) The coenzyme Q10 analog decylubiquinone inhibits the redox-activated mitochondrial permeability transition: role of mitochondrial [correction mitochondrial] complex III. J Biol Chem 278:49079–49084. doi:10.1074/jbc.M307841200
Bargou RC et al (1996) Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest 97:2651–2659. doi:10.1172/JCI118715
Chang CT et al (2017) Antrodia salmonea induces G2 cell-cycle arrest in human triple-negative breast cancer (MDA-MB-231) cells and suppresses tumor growth in athymic nude mice. J Ethnopharmacol 196:9–19. doi:10.1016/j.jep.2016.12.018
Chen Q, Gong B, Almasan A (2000) Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ 7:227–233. doi:10.1038/sj.cdd.4400629
Chen Y, Zhang H, Zhou HJ, Ji W, Min W (2016) Mitochondrial redox signaling and tumor progression. Cancers (Basel). doi:10.3390/cancers8040040
Chiara F et al (2008) Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS One 3:e1852. doi:10.1371/journal.pone.0001852
Chiara F et al (2012) Chemotherapeutic induction of mitochondrial oxidative stress activates GSK-3alpha/beta and Bax, leading to permeability transition pore opening and tumor cell death. Cell Death Dis 3:e444. doi:10.1038/cddis.2012.184
Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G (2002) Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett 512:334–340
Chu Y, Goldman JG, Kelly L, He Y, Waliczek T, Kordower JH (2014) Abnormal alpha-synuclein reduces nigral voltage-dependent anion channel 1 in sporadic and experimental Parkinson’s disease. Neurobiol Dis 69:1–14. doi:10.1016/j.nbd.2014.05.003
Chung C-H, Yeh S-C, Chen C-J, Lee K-T (2014a) Coenzyme Q0 from Antrodia cinnamomea in submerged cultures induces reactive oxygen species-mediated apoptosis in A549 human lung cancer cells. Evid Based Complement Altern Med 2014:10. doi:10.1155/2014/246748
Chung CH, Yeh SC, Chen CJ, Lee KT (2014b) Coenzyme Q0 from Antrodia cinnamomea in submerged cultures induces reactive oxygen species-mediated apoptosis in A549 human lung cancer cells. Evid Based Complement Altern Medicine eCAM 2014:246748. doi:10.1155/2014/246748
Devun F, Walter L, Belliere J, Cottet-Rousselle C, Leverve X, Fontaine E (2010) Ubiquinone analogs: a mitochondrial permeability transition pore-dependent pathway to selective cell death. PLoS One 5:e11792. doi:10.1371/journal.pone.0011792
Ding W, Hudson LG, Liu KJ (2005) Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol Cell Biochem 279:105–112. doi:10.1007/s11010-005-8227-y
Doughan AK, Dikalov SI (2007) Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9:1825–1836
Du H et al (2008) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med 14:1097–1105. doi:10.1038/nm.1868
Esaka Y, Nagahara Y, Hasome Y, Nishio R, Ikekita M (2005) Coenzyme Q2 induced p53-dependent apoptosis. Biochim Biophys Acta 1724:49–58
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411:342–348. doi:10.1038/3507721335077213
Farley N et al (2006) p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells. Mol Cell Biol 26:2118–2129. doi:10.1128/MCB.26.6.2118-2129.2006
Gall JM et al (2011) Hexokinase regulates Bax-mediated mitochondrial membrane injury following ischemic stress. Kidney Int 79:1207–1216. doi:10.1038/ki.2010.532
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Hseu YC et al (2016) In vitro and in vivo anti-tumor activity of CoQ0 against melanoma cells: inhibition of metastasis and induction of cell-cycle arrest and apoptosis through modulation of Wnt/beta-catenin signaling pathways. Oncotarget 7:22409–22426. doi:10.18632/oncotarget.79837983
Huang H, Shah K, Bradbury NA, Li C, White C (2014) Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis 5:e1482. doi:10.1038/cddis.2014.419
Jemiota Rzemińska M, Latowski D, Strzałka K (2001) Incorporation of plastoquinone and ubiquinone into liposome membranes studied by HPLC analysis: the effect of side chain length and redox state of quinone. Chem Phys Lipids 110:85–94
Jiang N, Kham SK, Koh GS, Suang Lim JY, Ariffin H, Chew FT, Yeoh AE (2011) Identification of prognostic protein biomarkers in childhood acute lymphoblastic leukemia (ALL). J Proteom 74:843–857. doi:10.1016/j.jprot.2011.02.034
Jung JY et al (2007) Epigallocatechin gallate inhibits nitric oxide-induced apoptosis in rat PC12 cells. Neurosci Lett 411:222–227. doi:10.1016/j.neulet.2006.09.089
Knudsen CA, Tappel AL, North JA (1996) Multiple antioxidants protect against heme protein and lipid oxidation in kidney tissue. Free Radic Biol Med 20:165–173
Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44:479–496. doi:10.3109/10715761003667554
Lu C, El-Deiry WS (2009) Targeting p53 for enhanced radio- and chemo-sensitivity. Apoptosis Int J Program Cell Death 14:597–606. doi:10.1007/s10495-009-0330-1
MacDonald MJ (1991) Stimulation of insulin release from pancreatic islets by quinones. Biosci Rep 11:165–170
Messina A, Reina S, Guarino F, De Pinto V (2012) VDAC isoforms in mammals. Biochim Biophys Acta 1818:1466–1476. doi:10.1016/j.bbamem.2011.10.005
Orrenius S (2007) Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev 39:443–455. doi:10.1080/03602530701468516
Park N, Baek HS, Chun YJ (2015) Embelin-induced apoptosis of human prostate cancer cells is mediated through modulation of Akt and beta-catenin signaling. PLoS One 10:e0134760. doi:10.1371/journal.pone.0134760
Pastorino JG, Hoek JB (2008) Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr 40:171–182. doi:10.1007/s10863-008-9148-8
Pieper AA, Verma A, Zhang J, Snyder SH (1999) Poly (ADP-ribose) polymerase, nitric oxide and cell death. Trends Pharmacol Sci 20:171–181
Pilz RB, Van den Berghe G, Boss GR (1987) Induction of HL-60 differentiation by starvation for a single essential amino acid but not by protein synthesis inhibitors. J Clin Invest 79:1006–1009. doi:10.1172/JCI112867
Qin LS, Jia PF, Zhang ZQ, Zhang SM (2015) ROS-p53-cyclophilin-D signaling mediates salinomycin-induced glioma cell necrosis. J Exp Clin Cancer Res 34:57. doi:10.1186/s13046-015-0174-1
Rasola A, Bernardi P (2007) The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis Int J Program Cell Death 12:815–833. doi:10.1007/s10495-007-0723-y
Robey RB, Hay N (2006) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25:4683–4696. doi:10.1038/sj.onc.1209595
Saedi TA, Md Noor S, Ismail P, Othman F (2014) The effects of herbs and fruits on leukaemia. Evid Based Complement Altern Med eCAM 2014:494136. doi:10.1155/2014/494136
Scherz-Shouval R, Elazar Z (2007) ROS, mitochondria and the regulation of autophagy. Trends Cell Biol 17:422–427. doi:10.1016/j.tcb.2007.07.009
Selimovic D, Hassan M, Haikel Y, Hengge UR (2008) Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell Signal 20:311–322. doi:10.1016/j.cellsig.2007.10.015
Sharaf el dein O, Gallerne C, Brenner C, Lemaire C (2012) Increased expression of VDAC1 sensitizes carcinoma cells to apoptosis induced by DNA cross-linking agents. Biochem Pharmacol 83:1172–1182. doi:10.1016/j.bcp.2012.01.017
Shipley JL, Butera JN (2009) Acute myelogenous leukemia. Exp Hematol 37:649–658. doi:10.1016/j.exphem.2009.04.002
Shoshan-Barmatz V, Ben-Hail D (2012) VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion 12:24–34. doi:10.1016/j.mito.2011.04.001
Somers-Edgar TJ, Rosengren RJ (2009) Coenzyme Q0 induces apoptosis and modulates the cell cycle in estrogen receptor negative breast cancer cells. Anticancer Drugs 20:33–40. doi:10.1097/CAD.0b013e328314b5c5
Tajeddine N et al (2008) Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death. Oncogene 27:4221–4232. doi:10.1038/onc.2008.63
Tanno M et al (2014) Translocation of glycogen synthase kinase-3beta (GSK-3beta), a trigger of permeability transition, is kinase activity-dependent and mediated by interaction with voltage-dependent anion channel 2 (VDAC2). J Biol Chem 289:29285–29296. doi:10.1074/jbc.M114.563924
Thiyagarajan V, Lin SH, Chia YC, Weng CF (2013) A novel inhibitor, 16-hydroxy-cleroda-3,13-dien-16,15-olide, blocks the autophosphorylation site of focal adhesion kinase (Y397) by molecular docking. Biochim Biophys Acta 1830:4091–4101. doi:10.1016/j.bbagen.2013.04.027
Thiyagarajan V, Tsai MJ, Weng CF (2015) Antroquinonol targets FAK-signaling pathway suppressed cell migration, invasion, and tumor growth of C6 glioma. PLoS One 10:e0141285. doi:10.1371/journal.pone.0141285
Thiyagarajan V, Sivalingam KS, Viswanadha VP, Weng CF (2016) 16-hydroxy-cleroda-3,13-dien-16,15-olide induced glioma cell autophagy via ROS generation and activation of p38 MAPK and ERK-1/2. Environ Toxicol Pharmacol 45:202–211. doi:10.1016/j.etap.2016.06.005
Tomasello F et al (2009) Outer membrane VDAC1 controls permeability transition of the inner mitochondrial membrane in cellulo during stress-induced apoptosis. Cell Res 19:1363–1376. doi:10.1038/cr.2009.98
Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM (2012) p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149:1536–1548. doi:10.1016/j.cell.2012.05.014
Vigneron F et al (2011) GSK-3beta at the crossroads in the signalling of heart preconditioning: implication of mTOR and Wnt pathways. Cardiovasc Res 90:49–56. doi:10.1093/cvr/cvr002
Vousden KH, Lane DP (2007) p53 in health and disease. Nat Rev Mol Cell Biol 8:275–283. doi:10.1038/nrm2147
Wagner EF, Nebreda AR (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 9:537–549. doi:10.1038/nrc2694
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–2933
Wang HM et al (2016) Coenzyme Q0 enhances ultraviolet B-induced apoptosis in human estrogen receptor-positive breast (MCF-7) cancer cells. Integr Cancer Ther. doi:10.1177/1534735416673907
Yang HL et al (2016a) Coenzyme Q0 regulates NFkappaB/AP-1 activation and enhances Nrf2 stabilization in attenuation of LPS-induced inflammation and redox imbalance: evidence from in vitro and in vivo studies. Biochim Biophys Acta 1859:246–261. doi:10.1016/j.bbagrm.2015.11.001
Yang HL et al (2016b) Toona sinensis inhibits murine leukemia WEHI-3 cells and promotes immune response in vivo. Integr Cancer Ther. doi:10.1177/1534735416642863
Yonezawa Y et al (2006) Inhibitory effect of coenzyme Q1 on eukaryotic DNA polymerase γ and DNA topoisomerase II activities on the growth of a human cancer cell line. Cancer Sci 97:716–723. doi:10.1111/j.1349-7006.2006.00236.x
Acknowledgements
This work was supported by the Grants MOST-104-2320-B-039-040-MY3, MOST-103-2320-B-039-038-MY3, and NSC-103-2622-B-039-001-CC2 from the Ministry of Science and Technology, National Science Council, Asia University and China Medical University, Taiwan. This study was supported by China Medical University under the Aim for Top University Plan of the Ministry of Education, Taiwan (CHM106-5-3).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Hseu, YC., Thiyagarajan, V., Ou, TT. et al. CoQ0-induced mitochondrial PTP opening triggers apoptosis via ROS-mediated VDAC1 upregulation in HL-60 leukemia cells and suppresses tumor growth in athymic nude mice/xenografted nude mice. Arch Toxicol 92, 301–322 (2018). https://doi.org/10.1007/s00204-017-2050-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-2050-6