Abstract
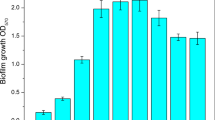
This study aims to describe the content of polymeric matrix components under different incubation temperatures and pH levels. Optimal biofilm production of 15 S. Virchow isolates occurred following the incubation in LB−NaCl for 72 h, at pH 6.6 and 20 °C. The expression of csgA, csgD, adrA and bcsA genes at 20 °C, 25 °C and 30 °C in S. Virchow DMC18 was analyzed, and it was discovered that the maximum production of cellulose and curli fimbriae occurred at 20 °C. The physical characteristics of pellicle structure of S. Virchow DMC18 was determined as rigid at 20 °C, while becoming fragile at higher temperatures. FTIR analyses confirmed the obtained molecular findings. The intensities of the 16 different peaks originating from carbohydrate, protein, and nucleic acid in the spectra of biofilm samples significantly diminished (p < 0.05) with the increasing temperature. The highest intensities of lipids and carbohydrates were observed at 20 °C indicating the changes in cell surface properties.










Similar content being viewed by others
References
Adiguzel Y, Haris PI, Severcan F (2012) Screening of proteins in cells and tissues by vibrational spectroscopy. In: Severcan F, Haris PI (eds) Chapter 3, screening of proteins in cell and tissues by vibrational spectroscopy, 1st edn. IOS Press, Amsterdam, pp 53–108
Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T (2006) A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128
Berne C, Kysela DT, Brun YV (2010) A bacterial extracellular DNA inhibits settling of motile progeny cells within a biofilm. Mol Microbiol 77:815–829
Borges KA, Furian TA, Souza SN, Menezes R, Tando EC, Salle CTP, Maraes HLS, Nascimento VP (2018) Biofilm formation capacity of Salmonella serotypes at different temperature conditions. Pesq Vet Bras 38:71–76
Brown PK, Dozois CM, Nickerson CA, Zuppardo A, Terlange J, Curtiss R 3rd (2001) MlrA, a novel regulator of curli (AgF) and extracellular matrix synthesis by Escherichia coli and Salmonella enterica serovar Typhimurium. Mol Microbiol 41:349–363
Castelijn GA, van der Veen S, Zwietering MH, Moezelaar R, Abee T (2012) Diversity in biofilm formation and production of curli fimbriae and cellulose of Salmonella Typhimurium strains of different origin in high and low nutrient medium. Biofouling 28:51–63
Centers for Disease Control and Prevention (2016) Multistate outbreak of Salmonella Virchow ınfections linked to garden of life raw meal organic shake & meal products (Final Update). https://www.cdc.gov/salmonella/virchow-02-16/index.html. Accessed 2016 Apr 21
Chandy JP, Angles ML (2001) Determination of nutrients limiting biofilm formation and the subsequent impact on disinfectant decay. Water Res 35:2677–2682
Chmielewski RAN, Frank JF (2003) Biofilm formation and control in food processing facilities. Compr Rev Food Sci Food Saf 2:22–32
Chu C, Doublet B, Lee YL, Cloeckaert A, Chiou CS, Chen SW, Lin CW, Chiu CH (2012) Salmonella genomic island 1-J variants associated with change in the antibiotic resistance gene cluster in multıdrug resistant Salmonella enterica serovar Virchow isolated from humans, Taiwan, 2004–2006. Clin Microbiol Infect 18:47–53
Conover MS, Mishra M, Deora R (2011) Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice. PLoS One 6:e16861
Costerton JW (1999) Introduction to biofilm. Int J Antimicrob Agents 11:217–221
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, LappinScott HM (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745
Crawford RW, Reeve KE, Gunn JS (2010) Flagellated but not hyperfimbriated Salmonella enterica serovar Typhimurium attaches to and forms biofilms on cholesterol-coated surfaces. J Bacteriol 192:2981–2990
Crump JA, Griffin PM, Angulo FJ (2002) Bacterial contamination of animal feed and its relationship to human foodborne illness. J Food Saf 35:859–865
Davey ME, O’Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867
European Centre for Disease Prevention and Control (2012) The European union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2010. EFSA J 10:2597
Finkel SE, Kolter R (2001) DNA as a nutrient: novel role for bacterial competence gene homologs. J Bacteriol 183:6288–6293
Garip S, Bozoglu F, Severcan F (2007) Differentiation of mesophilic and thermophilic bacteria with fourier transform infrared spectroscopy. Appl Spectrosc 61:186–192
Gerstel U, Römling U (2001) Oxygen tension and nutrient starvation are major signals that regulate agfD promoter activity and expression of the multicellular morphotype in Salmonella typhimurium. Environ Microbiol 3:638–648
Gerstel U, Römling U (2003) The csgD promoter, a control unit for biofilm formation in Salmonella Typhimurium. Res Microbiol 154:659–667
Gerstel U, Park C, Römling U (2003) Complex regulation of csgD promoter activity by global regulatory proteins. Mol Microbiol 49:639–654
Hall-Stoodley L, Nistico L, Sambanthamoorthy K, Dice B, Nguyen D, Mershon WJ, Johnson C, Hu FZ, Stoodley P, Ehrlich GD, Post JC (2008) Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule down regulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol 8:173. https://doi.org/10.1186/1471-2180-8-173
Hammar M, Arnqvist A, Bian Z, Olsen A, Normark S (1995) Expression of two csg operons is required for production of fibronectin-and congo red-binding curli polymers in Escherichia coli K-12. Mol Microbiol 18:661–670
Harmsen M, Lappann M, Knøchel S, Molin S (2010) Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Appl Environ Microbiol 76:2271–2279
Helfer GA, Bock F, Marder L, Furtado JC, Costa AB, Ferrão MF (2015) Chemostat: exploratory multivariate data analysis software. Quim Nova 38:575–579
Jensen PO, Givskov M, Bjarnsholt T, Moser C (2010) The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol 59:292–305
Karaca B, Akçelik N, Akçelik M (2013) Biofilm-producing abilities of Salmonella strains isolated from Turkey. Biologia 68:1–10
Kolter R, Greenberg EP (2006) Microbial sciences: the superficial life of microbes. Nature 441:300–302
Lappann M, Claus H, van Alen T, Harmsen M, Elias J, Molin S, Vogel U (2010) A dual role of extracellular DNA during biofilm formation of Neisseria meningitides. Mol Microbiol 75:1355–1371
Ledeboer NA, Jones BD (2005) Exopolysaccharide sugars contribute to biofilm formation by Salmonella enterica serovar Typhimurium on HEp–2 cells and chicken intestinal epithelium. J Bacteriol 187:3214–3226
Movasaghi Z, Rehman S, Rehman I (2008) Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl Spectrosc Rev 43:134–179
Mulcahy H, Charron-Mazenod L, Lewenza S (2010) Pseudomonas aeruginosa produces an extracellular deoxyribonuclease that is required for utilization of DNA as a nutrient source. Environ Microbiol 12:1621–1629
Paytubi S, Cansado C, Madrid C, Balsalobre C (2017) Nutrient composition promotes switching between pellicle and bottom biofilm in Salmonella. Front Microbiol 8:2160. https://doi.org/10.3389/fmicb.2017.02160
Piras F, Fois F, Consolati SG, Mazza R, Mazzette R (2015) Influence of temperature, source and serotype on biofilm formation of Salmonella enterica isolates from pig slaughterhouses. J Food Prot 78:1875–1878
Prouty AM, Schwesinger WH, Gunn JS (2002) Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun 70:2640–2649
Römling U, Rodhe M (1999) Flagella modulate the multicellular behavior of Salmonella Typhimurium on the community level. FEMS Microbiol Lett 180:91–102
Römling U, Sierralta WD, Eriksson K, Normark S (1998a) Multicellular and aggregative behaviour of Salmonella Typhimurium strains is controlled by mutations in the agfD promoter. Mol Microbiol 28:249–264
Römling U, Bian Z, Hammar M, Sierralta WD, Normark S (1998b) Curli fibers are highly conserved between Salmonella Typhimurium and E. coli with respect to operon structure and regulation. J Bacteriol 180:722–731
Römling U, Rodhe M, Olsen A, Normark S, Reinkoster J (2000) Agf D, the checkpoint of multicellular and aggregative behavior in Salmonella typhimurium regulates at least two independent pathways. Mol Microbiol 36:10–23
Römling U, Bokranz W, Rabsch W, Zogaj X, Nimtz M, Tschape H (2003) Occurrence and regulation of the multicellular morphotype in Salmonella serovars important in human disease. Int J Med Microbiol 293:273–285
Römling U, Persen D, Yaron S (2007) Biofilms of Salmonella enterica. In: Rhen M, Maskell D, Mastroeni P, Threlfall EJ (eds) Salmonella molecular biology and pathogenesis, Norfolk: 4K. Harizon Press, Chennai, pp 127–145
Sauer K, Rickard AH, Davies DG (2007) Biofilm and biocomplexity. Features 2:347–353
Schmitt J, Flemming HC (1998) FTIR-spectroscopy in microbial and material analysis. Int Biodeter Biodegr 41:1–11
Severcan F, Akkas SB, Turker S, Yucel R (2012) Methodological approaches from experimental to computational analysis in vibrational spectroscopy and microspectroscopy. In: Severcan F, Haris PI (eds) Chapter 2, screening of proteins in cell and tissues by vibrational spectroscopy. IOS Press, Amsterdam, pp 12–52
Shi X, Zhu X (2009) Biofilm formation and food safety in food industries. Trends Food Sci Technol 20:407–413
Simm R, Morr M, Kader A, Nimtz M, Römling U (2004) GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol 53:1123–1134
Simoes M, Simoes LC, Vieira MJ (2010) A review of current and emergent biofilm control strategies. LWT Food Sci Technol 43:573–583
Sinde E, Carballo J (2000) Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: the influence of free energy and the effect of commercial sanitizers. Food Microbiol 17:439–447
Solano C, Garcia B, Valle J, Berasain C, Ghigo JM, Gamazo C, Lasa I (2002) Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol Microbiol 43:793–808
Solomon EB, Niemira BA, Sapers GM, Annous BA (2005) Biofilm formation, cellulose production, and curli biosynthesis by Salmonella originating from produce, animal, and clinical sources. J Food Protect 68:906–912
Spoering AL, Gilmore MS (2006) Quorum sensing and DNA release in bacterial biofilms. Curr Opin Microbiol 9:133–137
Spoering AS, Lewis K (2001) Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobial. J Bacteriol 183:6746–6751. https://doi.org/10.1128/JB.183.23.6746-6751.2001
Steenackers H, Hermans K, Vanderleyden J, De Keersmaecker SCJ (2012) Salmonella biofilms: an overview on occurrence, structure, regulation and eradication. Food Res Int 45:502–531
Stepanovic S, Cirkovic I, Mijac V, Svabic-Vlahovic M (2003) Influence of the incubation temperature, atmosphere and dynamic conditions on biofilm formation by Salmonella spp. Food Microbiol 20:339–343
Stepanovic S, Cirkovic I, Ranin L, Svabic-Vlahovic M (2004) Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett Appl Microbiol 38:428–432
Teiveira P, Lima JC, Azeredo J, Oliveira R (2007) Note. Colonisation of bench cover materials by Salmonella Typhimurium. Food Sci Tech Int. https://doi.org/10.1177/1082013207075157
Teplitski M, Barak JD, Schneider KR (2009) Human enteric pathogens in produce: un-answered ecological questions with direct implications for food safety. Curr Opin Biotechnol 20:166–171
Vestby LK, Moretro T, Langsrud S, Heir E, Nesse LL (2009) Biofilm forming abilities of Salmonella are correlated with persistence in fish meal- and feed factories. BMC Vet Res 5:20–26
Wang H, Huang Y, Shuyan W, Li Y, Ye Y, Zheng Y, Huang R (2013) Extracellular DNA inhibits Salmonella enterica serovar Typhimurium and S. enterica serovar Typhi biofilm development on abiotic surfaces. Curr Microbiol 68:262–268
Weinberger M, Solnik-Isaac H, Shackar D, Reisfeld A, Valinsky L, Andorn N, Agmon V, Yishai R, Bassal R, Fraser A, Yaron S, Cohen D (2006) Salmonella enterica serotype Virchow: epidemiology, resistance patterns and molecular characterization of an invasive Salmonella serotype in Israel. Clin Microbiol Infect 12:999–1005
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295:1487
White AP, Gibson DL, Kim W, Kay WW, Surette MG (2006) Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella. J Bacteriol 188:3219–3227
White AP, Gibson DL, Grassl GA, Kay WW, Finlay BB, Vallance BA, Surette MG (2008) Aggregation via the red, dry and rough morphotype is not a virulence adaptation in Salmonella enterica serovar Typhimurium. Infect Immun 76:1048–1058
Yener İlçe B, Akçelik N (2015) The effect of marT gene on biofilm production of Salmonella Typhimurium. Turk J Biol. 39:722–731
Zogaj X, Bokranz W, Nimtz M, Römling U (2003) Production of cellulose and curli fimbriae by members of the family enterobacteriaceae isolated from the human gastrointestinal tract. Infect Immun 71:4151–4158
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Author contributions
NA: coordinated and drafted the manuscript, carried out the experiments. Nİ: performed the FTIR experiments and participated in the interpretation of the FTIR results. NA* and MA conceived of the study and planned the experiments, supervised the project and took the lead in the writing the manuscript. All the authors provided critical feedback and helped the research, analysis and manuscript.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ariafar, M.N., Iğci, N., Akçelik, M. et al. Investigation of the effect of different environmental conditions on biofilm structure of Salmonella enterica serotype Virchow via FTIR spectroscopy. Arch Microbiol 201, 1233–1248 (2019). https://doi.org/10.1007/s00203-019-01681-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-019-01681-5