Abstract
The obligate intracellular bacterium, Wolbachia pipientis (Rickettsiales), is a widespread, vertically transmitted endosymbiont of filarial nematodes and arthropods. In insects, Wolbachia modifies reproduction, and in mosquitoes, infection interferes with replication of arboviruses, bacteria and plasmodia. Development of Wolbachia as a tool to control pest insects will be facilitated by an understanding of molecular events that underlie genetic exchange between Wolbachia strains. Here, we used nucleotide sequence, transcriptional and proteomic analyses to evaluate expression levels and establish the mosaic nature of genes flanking the T4SS virB8-D4 operon from wStr, a supergroup B-strain from a planthopper (Hemiptera) that maintains a robust, persistent infection in an Aedes albopictus mosquito cell line. Based on protein abundance, ribA, which contains promoter elements at the 5′-end of the operon, is weakly expressed. The 3′-end of the operon encodes an intact wspB, which encodes an outer membrane protein and is co-transcribed with the vir genes. WspB and vir proteins are expressed at similar, above average abundance levels. In wStr, both ribA and wspB are mosaics of conserved sequence motifs from Wolbachia supergroup A- and B-strains, and wspB is nearly identical to its homolog from wCobU4-2, an A-strain from weevils (Coleoptera). We describe conserved repeated sequence elements that map within or near pseudogene lesions and transitions between A- and B-strain motifs. These studies contribute to ongoing efforts to explore interactions between Wolbachia and its host cell in an in vitro system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wolbachia pipientis (Rickettsiales; Alphaproteobacteria) is an obligate intracellular bacterium that infects filarial nematodes and a wide range of arthropods including ≥60 % of insects and ≈35 % of isopod crustaceans, but does not infect vertebrates (Hilgenboecker et al. 2008). Wolbachia is considered to be a single species classified into clades by multilocus sequence typing and designated as supergroups A to N (Baldo et al. 2006b; Comandatore et al. 2013; Lo et al. 2007). The C- and D-strains that infect filarial worms have phylogenies concordant with those of nematode hosts, consistent with strict vertical transmission as obligate mutualists (Comandatore et al. 2013; Dedeine et al. 2003; Li and Carlow 2012; Strubing et al. 2010; Taylor et al. 2005; Wu et al. 2004). Although arthropod-associated A- and B-strains may provide subtle fitness benefits to hosts (Zug and Hammerstein 2014), they are best known as reproductive parasites, causing phenotypes that maintain or increase Wolbachia infection frequencies, including feminization, parthenogenesis, and cytoplasmic incompatibility (Saridaki and Bourtzis 2010; Werren et al. 2008). Interference with host immune mechanisms and replication of arboviruses, bacteria and malarial plasmodia (Kambris et al. 2009; Pan et al. 2012; Zug and Hammerstein 2014) has encouraged efforts to exploit Wolbachia for biocontrol of arthropod vectors of vertebrate pathogens and/or crop pests (Bourtzis 2008; Rio et al. 2004; Sinkins and Gould 2006; Zabalou et al. 2004). An understanding of molecular differences between A- and B-strains, and how they have been influenced by horizontal transmission and genetic exchange (Newton and Bordenstein 2011; Schuler et al. 2013; Werren et al. 2008; Zug and Hammerstein 2014) will facilitate manipulation of Wolbachia.
Wolbachia’s interaction with host cells likely involves the type IV secretion system (T4SS), a macromolecular complex that transports DNA, nucleoproteins and “effector” proteins across the microbial cell envelope into the host cell, where they mediate intracellular interactions (Alvarez-Martinez and Christie 2009; Zechner et al. 2012). Homologs of all genes except virB5 of Agrobacterium tumefaciens T4SS have been identified in Wolbachia and other members of the Rickettsiales (Gillespie et al. 2009, 2010), including Anaplasma, Ehrlichia, Neorickettsia, Orientia and Rickettsia. Among sequenced Wolbachia genomes, T4SS genes are organized in two operons: virB3-B6 containing virB3, virB4 and four virB6 paralogs and virB8-D4 containing virB8, virB9, virB10, virB11, virD4 and, in some genomes, the wspB paralog of the wspA major surface antigen (Pichon et al. 2009; Rances et al. 2008). In the supergroup B-strain wPip from Culex pipiens mosquitoes, wspB is disrupted by a transposon and is presumably inactive (Sanogo et al 2007). T4SS effector proteins that manipulate host cells have been identified from Anaplasma and Ehrlichia (Liu et al. 2012; Lockwood et al. 2011; Niu et al. 2010), and Wolbachia express both vir operons in ovaries of arthropod hosts, wherein T4SS effectors are suspected to play a role in cytoplasmic incompatibility and other reproductive distortions (Masui et al. 2000; Rances et al. 2008; Wu et al. 2004). Although WspA and WspB are likely components of the Wolbachia outer membrane, their functions remain unknown. In the case of wBm, WspB is excreted/secreted into filarial host cells (Bennuru et al. 2009) and co-localizes with the Bm1_46455 host protein in tissues that include embryonic nuclei (Melnikow et al. 2011). WspB is therefore itself a candidate T4SS effector that may play a role in reproductive manipulation of the host.
The Wolbachia strain wStr in supergroup B causes strong cytoplasmic incompatibility in the planthopper, Laodelphax striatellus (Noda et al. 2001a), and in addition maintains a robust, persistent infection in a clonal Aedes albopictus mosquito cell line, C/wStr1 (Fallon et al. 2013; Noda et al. 2002). Because in vitro studies with wStr provide advantages of scale and ease of manipulation for exploring mechanisms that may facilitate transformation and genetic manipulation of Wolbachia, we have undertaken proteomics-based studies that provide strong support for expression of T4SS machinery in cell culture. Here, we report the sequence of the virB8-D4 operon, including flanking genes ribA, upstream of virB8, and wspB downstream of virD4. We show that wspB is intact, describe protein structure predicted from the deduced WspB sequence, and verify co-transcription of wspB with upstream vir genes. Relative abundance levels of WspB and the VirB8-D4 proteins in wStr are well above average, while RibA is among the least abundant of MS-detected proteins. In wStr, ribA and wspB are mosaics of sequence motifs that are differentially conserved in supergroup A- (WOL-A) and B- (WOL-B) strains, and they contain conserved 8-bp repeat elements that may be associated with genetic exchange. Finally, we discuss implications for functional integration of the Wolbachia T4SS with WspB and with the riboflavin biosynthesis pathway enzymes GTP cyclohydrolase II (RibA) and dihydroxybutanone phosphate synthase (RibB).
Materials and methods
Cultivation of cells
Aedes albopictus C7-10 and C/wStr1 cells were maintained in Eagle’s minimal medium supplemented with 5 % fetal bovine serum at 28–30 °C in a 5 % CO2 atmosphere (Fallon et al. 2013; Shih et al. 1998). Cells were harvested during exponential growth, under conditions favoring maximal recovery of Wolbachia (Baldridge et al. 2014).
Polymerase chain reaction, cloning and DNA sequencing
The polymerase chain reaction (PCR) was used to amplify wStr genes from DNA extracts prepared from Wolbachia enriched by fractionation of C/wStr1 cells on sucrose density gradients and recovered from the interface between 50 and 60 % sucrose (Baldridge et al. 2014). Template DNA was used to obtain 21 PCR products using a panel of 31 primers (Table S1), GoTaq™ DNA polymerase (Promega, Madison, WI), and a Techne TC-312 cycler (Staffordshire, UK). Cycle parameters were: 1 cycle at 94 °C for 2 min, 35 cycles at 94 °C for 35 s, 53 °C for 35 s, 72 °C for 1 min, followed by 1 cycle at 72 °C for 5 min. Extension time was increased to 2 min for products ≥1000 bp. PCR products were cloned in the pCR4-TOPO vector with the TOPO-TA Cloning Kit for Sequencing (Life Technologies, Grand Island, NY), and two or more clones each were sequenced at the University of Minnesota BioMedical Genomics Center.
Reverse transcriptase polymerase chain reaction
Total RNA was purified from A. albopictus C7-10 and C/wStr1 cells using the PureLink RNA Mini Kit (Life Technologies) and treated with DNase I (RNase-free; Life Technologies) followed by heat inactivation, as suggested by the manufacturer. RT-PCR was executed with primers virD4F1764–1784 and wspBR152–172 (Table S1) using the RNA PCR Core Kit (Life Technologies) as suggested by the manufacturer with the exception that synthesized cDNA was treated with DNase-inactivated RNaseA before the final PCR reaction. The PCR reaction included 1 cycle at 95 °C for 4 min, 35 cycles at 95 °C for 35 s, 56 °C for 40 s, 72 °C for 40 s, followed by 1 cycle at 72 °C for 3 min. Reaction products were electrophoresed on 1 % agarose gels, cloned, and sequenced as above.
Sequence alignments and protein structure prediction
DNA and protein sequence alignments were executed with the Clustal Omega program (Sievers et al. 2011). Alignments were edited by visual inspection and modified in Microsoft Word. WspB protein structure predictions were obtained using tools available at www.predictprotein.org, including the PROFtmb program (Dell et al. 2010) for prediction of bacterial transmembrane beta barrels (Bigelow et al. 2004) and per-residue prediction of up-strand, down-strand, periplasmic loop and outer loop positions of residues. The PROFisis program (Ofran and Rost 2006) was used to predict WspB amino acid residues that are potentially involved in protein–protein interactions. Trees were produced using PAUP* version 4 (Swofford 2002). Amino acids were aligned with Clustal W, using pairwise alignment parameters of 25/0.5 and multiple alignment parameters of 10/0.2 for gap opening and gap extension, respectively. The protein weight matrix was set to Gonnet. The alignment was saved as a nexus file and loaded into PAUP*, and the trees were created using a heuristic search with the criterion set to parsimony. Bootstrap 50 % majority-rule consensus trees are based on 1000 replicates, with wBm (WOL-D) as the outgroup.
Mass spectrometry, peptide detection, protein identification and statistical analysis
Mass spectrometry data, generated using LC–MS/MS on LTQ and Orbitrap Velos mass spectrometers as four data sets, were described previously (Baldridge et al. 2014). The MS search database was modified to include deduced ORFs from wStr sequence data described herein. All tests of association were performed with SAS version 9.3 (Cary, NC; http://www.sas.com/en_us/home.html/).
Results
Structure of the wStr virB4-D8 operon
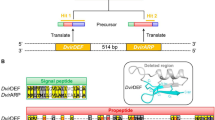
The robust, persistent infection of A. albopictus mosquito cell line, C/wStr1 with B wStr (in the text below, strain designations are denoted by superscripts), isolated from the planthopper L. striatellus, provides an in vitro model to identify proteins that modulate the host–microbe interaction. A potential role for the T4SS is supported by strong representation of peptides from VirB8, VirB9, VirB10, VirB11, VirD4 (Table 1) and associated proteins in the B wStr proteome (Baldridge et al. 2014). Despite its emergence as a useful strain that grows well in vitro, the B wStr genome is not yet available. In Wolbachia strains for which genome annotation is available, gene order within the virB8-D4 operon is conserved. Based on transcriptional analyses in the related genera, Anaplasma and Ehrlichia (Pichon et al. 2009), the promoter likely maps within the 3′-end of ribA extending into the intergenic spacer (Fig. 1a, black horizontal arrow at left) and is followed by five consecutive vir genes (Fig. 1b). In B wPip from Culex pipiens mosquitoes, wspB is disrupted by insertion of an IS256 element that encodes a transposase on the opposite strand (Fig. 1a, at right; Sanogo et al. 2007). Because VirB8-D4 proteins were highly similar to homologs from B wPip (Baldridge et al. 2014), we evaluated wspB in B wStr and its potential expression as a virB8-D4 operon member, as is the case in A wMel and A wRi from Drosophila spp. and A wAtab 3 from the wasp Asobara tabida (Rances et al. 2008; Wu et al. 2004). In the original proteomic analysis, three WspB peptides (Fig. 1a, tall black and gray arrows represent 95 and 94 % confidence peptides, respectively) mapped proximal and distal to the transposon insertion in B wPip, while the absence of peptides corresponding to the transposon suggested that wspB is intact in B wStr.
Schematic map of the Wolbachia T4SS virB8-D4 operon and cloning strategy for the ribA to topA sequence from B wStr. a Left expanded view of the B wStr ribA ORF depicted as an arrow showing the direction of transcription. Black horizontal arrow indicates a putative promoter that extends into an intergenic spacer (black rectangle). Black arrowheads indicate positions of MS-detected unique peptides (95 % confidence). Gradient shading from white to black designates 5′-sequence identity resembling WOL-A transitioning to 3′-sequence more closely resembling WOL-B-strains. a Right expanded view of the interrupted wspB homolog in B wPip. Black ellipses indicate positions of IS256 inverted repeat elements flanking a 1.2-kb insertion encoding a MULE domain superfamily transposase (gi|190571636; pfam10551) on the opposite strand (indicated by the direction of the open arrow); flanking gray shading indicates wspB. Tall vertical black and gray arrowheads indicate positions of unique peptides (95 and 94 % confidence, respectively) identified in the original MS data search. Small gray arrows indicate 95 % confidence peptides matched in a refined data set (including the B wStr sequence described here) that are conserved in WOL-B-strains, and open arrowheads with stars indicate peptides unique to B wStr. b Schematic depiction of the Wolbachia virB8-D4 operon and flanking genes with arrows designating the direction of transcription. Vir genes are designated in white font on a black background; black squares indicate intergenic spacers. Gradient shading indicates mosaic structure of an intact wspB in B wStr. c Filled lines above the 10-kb scale marker represent cloned PCR amplification products (see Table S1 for primers) that were sequenced and assembled into the B wStr ribB and ribA–topA consensus sequence. The double slash symbols at left indicate that ribB is not contiguous with downstream genes. The open box indicates the RT-PCR amplification product from Fig. 2. d BLASTn alignment of the 9133-bp B wStr ribA–topA sequence to corresponding sequences in B wVitB B wPip, B wVulC, A wRi, A wMel and D wBm genomes. Dark filled lines indicate sequence identity >70 %; light lines indicate low sequence identity, and the open space in B wPip represents an alignment gap
Nucleotide and deduced amino acid sequence comparisons
To examine the virB4-D4 operon in B wStr, we sequenced overlapping PCR products from 20 primer pairs (Table S1) spanning 9.1 kb beginning 43 bp downstream of the 5′-end of ribA in other Wolbachia strains and ending within topA encoded immediately downstream of the operon on the opposite strand (Fig. 1b, c). With the notable exception of the B wPip transposon, the nucleotide sequence aligned most closely to homologous sequences from B wVitB and B wPip. In addition, we noted variability in an ~0.3-kb region of virB10 in B wStr that was conserved in B wVitB, B wPip and A wRi, but not in B wVulC, A wMel and D wBm (Fig. 1d; see Table S2 for GenBank Accessions).
Pairwise sequence comparisons of the virB8-D4 operon from B wStr to homologs from Wolbachia supergroup A, B, C, D and F strains (Table 2) confirm that virB10, with nucleotide identities ranging from 74–99 %, is the least conserved of the five vir genes, and we note that Klasson et al. (2009) attributed divergence of virB10 in A wMel and A wRi to genetic exchange with a WOL-B-strain. Collectively and as individuals, the vir genes from B wStr have the highest nucleotide identities (~99 %) with B wVitB and B wPip. Identities with five A-strains are lower (range 87–91 %), lower yet (range 80–89 %) with the F-strain, F wCle and fall to a range of 74–88 % with three nematode-associated strains, D wBm, C wOo and C wOv. At the 5′-end of the operon, ribA was distinct, with approximately equivalent nucleotide identity with homologs from A- and B-strains (range 91–94 %), while the partial sequence of topA downstream of the operon had a conservation pattern similar to that of the vir genes. In some comparisons, virB8, virB11, virD4 and topA amino acid identities exceed nucleotide identities. Although ribB is not physically adjacent to the virB8-D4 operon in annotated Wolbachia genomes, ribB from B wStr is most similar to homologs from B wNo (97 % nucleotide identity) and A wMel (90 %), but was exceptional because identities with three other insect-associated A- and B-strains (~80 %) were lower than with F-, C- and D-strains (range 85–87 %). Consistent with earlier proteomic data (Baldridge et al. 2014), in all comparisons that discriminate between A- and B-strains, B wStr resembled WOL-B, while variability in ribA and wspB flanking the virB8-D4 genes exceeded that of the vir genes themselves.
Expression and relative abundances of the B wStr virB4-D8 proteins
To refine an earlier original proteomic analysis (Baldridge et al. 2014), we incorporated the PCR-amplified B wStr sequences described here to the database for peptide identification [Table 1, see column labeled Pep(2)]. Statistical analysis indicated that in a univariable model, protein molecular weight was weakly (r 2 = 0.2221) but significantly (p < 0.0001) associated with peptide count: log(peptides) = −0.40247 + 0.4953 × log(MW). Estimations of protein relative abundance levels (RAL) based on peptide counts were therefore normalized to protein length using studentized residuals (SR), a measure of deviance from expected values adjusted for estimated SD from the mean. All peptide data and SR values in the univariable and multivariable models of the original and refined searches are detailed in Table S3.
In the refined search, we identified eight new peptides from Vir proteins [Table 1, compare columns labeled Pep(2) to Pep(1)], including three from the most divergent VirB10. In aggregate, the five Vir proteins had a mean (SD) SR of 0.73 (0.2) and are expressed at above average abundance. We identified five new peptides from RibB, but none from RibA (Table 1). RibB has an SR of 1.2 and is an abundant protein, while RibA has an SR of −2.3 and is among the least abundant of MS-detected proteins. Nine new peptides from the highly divergent WspB (see below) generated an SR of 1.08, slightly above the threshold (>1.0) for an abundant protein and roughly equivalent to SR values (range 1–1.17) of housekeeping proteins such as isocitrate dehydrogenase, ftsZ, ATPsynthase F0F1 α subunit, and ribosomal proteins S2, S9, L3, L7/L12 and L14 (Table S3). In comparison, WspA with an SR of 2.17 (Table S3, entry 63) ranked as highly abundant, and the most abundant protein in the proteome was the GroEL chaperone (entry 586), with an SR of 3.66.
Reverse transcriptase PCR confirms co-transcription of wspB with vir genes
Similar SR values for WspB, relative to VirB8-D4, were consistent with evidence that wspB is co-transcribed with virB8-D4 in A wMel, A wRi and A wAtab 3 (Rances et al. 2008; Wu et al. 2004). We used RT-PCR with RNA template verified by PCR to be free of DNA contamination (Fig. 2b, lanes 2 and 3) to amplify a 528-bp product that was produced in reactions containing RNA from C/wStr1 cells (Fig. 2a, lane 4), but not in negative control reactions (lanes 1 and 2) or those with RNA from C7-10 cells (lane 3). Its sequence matched the expected B wStr genomic sequence (Fig. 1c, RT-PCR box at right), confirming that in B wStr, wspB is a member of the virB8-D4 operon.
Reverse transcriptase PCR (RT-PCR) analysis shows co-transcription of wspB with virD4. a Lanes 1 and 2 RT-PCR negative controls with no RNA or with no reverse transcriptase, respectively. Lanes 3 and 4 RT-PCR of RNA from uninfected C7-10 and infected C/wStr1 cells, respectively, with virD4 forward and wspB reverse primers. Lane 5 RT-PCR positive control with C/wStr1 RNA and Wolbachia primers S12F/S7R, which amplify portions of a ribosomal protein operon described previously (Fallon 2008). b Lane 1 PCR negative control with no Taq enzyme. Lanes 2 and 3 negative control lacking RT, with RNA from uninfected C7-10 and infected C/wStr1 cells, respectively
In B wStr, ribA is a mosaic of conserved WOL-A and WOL-B sequence motifs
The ribA nucleotide sequence has been shown to contain regulatory elements for expression of the T4SS operon in Anaplasma and Ehrlichia (Ohashi et al. 2002; Pichon et al. 2009). In contrast to highest homologies of B wStr virB8-D4 genes to WOL-B-strains, ribA sequence identities showed little difference between WOL-A and -B homologs (Table 2), but the two MS-detected peptides corresponded to A wMel and B wPip homologs, respectively (Fig. 1a). Alignment of amino acids from 10 RibA homologs (Fig. 3; WOL-A and WOL-B-strains are identified at left in red and blue, respectively) suggested that B wStr RibA is a two-part mosaic, each containing a protein functional domain.
Amino acid sequence alignment of RibA homologs from B wStr and Wolbachia supergroups A (red), B (blue) and D (black) respectively. Asterisks below the alignment indicate universally conserved residues. Unique residues are in green font. Residues conserved in B wStr and a majority of B-strains are in dark blue, bold font, while those in dark red, bold font are conserved with a majority of A-strains. Residues conserved in two to four strains are in light blue, orange or orange bold font. Residues highlighted in gray correspond to 95 % confidence peptides detected by LC–MS/MS. The dihydroxybutanone phosphate synthase (RibB) and GTP cyclohydrolase II domains (RibA) are indicated above the alignment within greater than less than symbols. Bold underlined residues in A wMel and B wStr indicate conserved active site amino acids, including critical cysteine residues. Double underlined residues indicate amino acids involved in the dimerization interface. See Tables 2 and S2 for host associations and GenBank Accessions. The PCR-amplified B wStr sequence does not encode the N-terminal amino acids; position 1 corresponds to the 15th amino acid
The amino terminal 150 residues in B wStr RibA (Fig. 3) include a short dihydroxybutanone phosphate synthase domain and the first detected peptide (residues 94–104). This portion of B wStr RibA matched sequences from the four A-strains and a single B-strain, B wVulC, at 29 of 36 variable amino acids (shown in red), while only three (4, 39 and 168 in blue) matched the other three B-strains and four (in green) were unique. In contrast, the C-terminal 151–347 residues, encompassing the second peptide (residues 250–258) within a GTP cyclohydrolase domain, included a single amino acid unique to B wStr, while 23 (in blue) uniformly matched B-strains except B wVulC, which continued to resemble the A-strains until residue 239. Among the four A-strains, the B wRi homolog is most similar throughout the alignment to the B-strains, but within residues 129–150 immediately preceding the cyclohydrolase domain, it closely matched B wTai, B wPip and B wVitB, while B wStr and B wVulC matched the other three A-strains. In aggregate, the alignment suggested that the B wStr and B wVulC homologs are two-part mosaics, each containing a protein functional domain, with an N-terminal WOL-A motif and a C-terminal WOL-B motif. We note that the C-terminal B-strain motif is consistent with the B-strain identity of the downstream virB8-D4 operon (Table 2) and includes the predicted promoter region (Ohashi et al. 2002; Pichon et al. 2009). Likewise, in a phylogenetic comparison (Fig. 4), trees representing the full length and N-terminal regions (top and bottom left) show B wVulC and B wStr in adjacent positions, and grouped more closely with WOL-A-strains. In the C-terminus, where the amino acid alignment shows an overall higher consensus (Fig. 3), B wStr grouped with the B-strains including B wPip, while B wVulC appears more closely related to A-strains.
Phylogenic relationships of B wStr RibA protein with homologs from WOL-A- and WOL-B-strains. Consensus trees show bootstrap values based on 1000 replicates, with D wBm (WOL-D) as the outgroup. WOL-A-strains are shown in black font boxed against a white background. WOL-B-strains are shown in white font on a black background. Open arrows designate BwVulC and closed arrows indicate B wStr. The N-terminal alignment corresponded to the first 150 residues in Fig. 3; the remainder of the protein was included in the C-terminal alignment
Nucleotide alignment and phylogenetic comparisons show that ribA is a mosaic gene in B wStr and B wVulC
A nucleotide alignment (Fig. S1) confirmed that ribA from B wStr is a two-part mosaic of WOL-A and WOL-B sequence motifs that correspond to the N- and C-terminal halves of the protein. In the first 522 nucleotides of ribA, 45 (in red font) of 56 variable nucleotides in B wStr match the A-strain sequences (Fig. S1), but only six (in blue) match the majority of B-strains and two are unique to B wStr (in green). In the downstream 522 nucleotides of ribA, 51 (in blue) of 54 variable nucleotides in B wStr match B-strains, while a single nucleotide (684 in red) matches the A-strains and two (in green) are unique to B wStr. In B wVulC, ribA has a similar two-part mosaic structure but does not firmly transit from the WOL-A to the WOL-B sequence motif until position 775, consistent with the amino acid alignment. Among the A-strains, ribA from A wRi is again most similar to the B-strain sequences. Within nucleotides 387–453 encoding amino acids 129–150 just before the cyclohydrolase domain and the A/B-strain sequence motif transition in B wStr, 13 of 18 WOL-A/B variable nucleotides in A wRi are shared with B wTai, B wPip and B wVitB, but those of B wStr and B wVulC are conserved with the other A-strains (orange and black vs. red residues, respectively).
WspB in B wStr is strikingly similar to a A wCobU4-2 homolog
Having shown that wspB is intact in B wStr, we mapped 11 peptides onto amino acid sequences encoded by 12 homologs (Fig. 5), including sequences deduced from three open reading frames (ORFs) in the wspB pseudogene from B wPip (Sanogo et al. 2007) and two overlapping ORFs in a pseudogene from A wCobU4-2, one of several WOL-A variants associated with the weevil, Ceutorhynchus obstrictus. Of two B wStr peptides (Fig. 5) detected at 95 % confidence in the original search (Baldridge et al. 2014), the first (residues 105–115 in gray) was identical in all strains except B wNo, which has unique M/I and V/I substitutions (residues in green). The second peptide (residues 209–220) is identical in all but the two A wCob strains that share an M/R substitution (215 in orange), while A wCobU4-2 has a unique Y/C substitution (219 in green). Five additional B wStr peptides (highlighted in cyan) were identical with B wVitB and B wMet (residues in blue), but not with B wPip and BwNo, which have many residues that are unique (in green) or shared (in orange) only with A wCobU5-2 and A wAna. Thus, with the exception of A wCobU5-2, cyan peptides of B wStr match other WOL-B-strains.
Amino acid sequence alignment of WspB homologs. At left, font color designates WOL-A (red) and B (blue) strains, and the B wStr sequence is the top listed Wol-B-strain. Asterisks below alignment indicate universally conserved residues; three hypervariable regions (HVRs) are doubly underlined above the alignment. Blocks of coloring designate peptides detected by LC–MS/MS at the 95 % confidence level. Those in gray were conserved in A- and B-strains. Cyan designates peptides conserved in B-strains, and yellow, those conserved in B wStr and A wCobU4-2. Olive peptides were unique to B wStr. Residues conserved between B wStr and a majority of A-strains are in red font (a single proline at residue 193) and residues conserved with a majority of B-strains are in blue font. Unique residues are in green font, and residues conserved between two or three homologs are in orange font. Underlined residues below the alignment denote the breakpoints between contiguous peptides within sequence regions. The greater than and less than symbols below the alignment indicate a transposon insertion in the wspB pseudogene of B wPip, followed by two additional deduced ORFs—see Fig. S2. PROFtmb (prediction of transmembrane beta barrels) symbols for individual residues below the alignment are: U—up-strand, D—down-strand, I—periplasmic loop, O—outer loop. PROFisis (prediction of protein–protein interaction residues) symbol P designates interaction residues. Wolbachia strain host associations: A wAtab 3, A. tabida—wasp; A wCob, C. obstrictus—weevil; B wMet, Metaseiulus occidentalis—predatory mite. See Tables 2 and S2 for other host associations and GenBank Accessions. The first 20 residues of theA wCob and B wMet sequences are not available
Two peptides underscore a striking similarity between the B wStr and A wCobU4-2 homologs. The first (Fig. 5, residues 133–140 highlighted in yellow) contains an alanine residue (138 in bold orange) shared only with A wCobU4-2. The second (residues 169–186 highlighted in olive) has a unique F/L substitution (in green) and a V/I substitution (in orange) shared with A wCobU4-2 and A wAna. Overall, the B wStr and A wCobU4-2 sequences differ at only five residues (59, 172, 193, 215 and 219), of which four occur within hypervariable regions. Throughout the alignment, A wAtab 3, A wKue, A wMel and A wRi form a conserved group, but the divergent A wAna and A wCobU4-2 and U5-2 strains have multiple residues (in blue, as in 42–77 and 224–277) that are conserved with the B-strains, suggesting genetic exchange between supergroups.
WspB domain structure and hypervariable regions (HVRs)
WspB is a paralog of the better-known WspA major surface antigen, which is anchored in the cell envelope by a transmembrane β-barrel domain (Koebnik et al. 2000), while surface-exposed loop domains contain HVRs with high recombination frequencies within and between strains (Baldo et al. 2010). The PROFtmb program predicted 10 transmembrane down (D)- and up (U)-strands and six periplasmic space (I) strands in WspB from B wStr (Fig. 5; residues indicated by D, U and I, respectively; Z score of 6.8 supports designation as transmembrane β-barrel protein). HVR1 and HVR2 each contain a predicted outer loop (residues 38–86 and 115–156 indicated by O) with high proportions of amino acids that are potentially charged at physiological pH; HVR3 contains two outer loops. Finally, a small predicted loop that is not within an HVR contains a proline (residue 193) that is conserved in B wStr and four WOL-A-strains. It is one of the 20 amino acids, most with hydrophilic or potentially charged side chains and within HVRs or adjacent to periplasmic space strands, predicted by the PROFisis program to be potentially involved in protein–protein interactions (P below alignment).
HVR1 amino acids
In HVR1 (Fig. 5, residues 41–77), eight residues are universally conserved among all homologs, while the majority of variable residues are differentially conserved in the B-strains (residues in blue) versus the A-strains. However, the sequences from the A wAna and A wCobU5-2 A-strains are mosaics in which eight of the first 20 residues (in blue) are conserved with all B-strains, while eight others are either conserved mutually or with B wNo or B wPip (in orange). Within the remaining 17 residues of HVR1, the A wAna and A wCobU5-2 sequences are better conserved with the other A-strains, while B wNo and B wPip have multiple unique residues (in green). The A wCobU4-2 and B wStr sequences differ only at residue 59.
HVR2 amino acids
Within HVR2 (Fig. 5, residues 121–150), A wCobU5-2 and A wAna sequences have alignment gaps at four residues, five or six unique residues respectively (in green), and eight residues that are either conserved mutually (in orange) or with B wNo. The B wPip pseudogene has only the first two residues of HVR2 due to a transposon insertion (indicated below alignment by greater than less than symbols). The A wCobU4-2 pseudogene contains a nucleotide sequence duplication (see below) that results in an overlap of the first and third ORFs beginning at the seventh residue of HVR2, but their spliced sequences, as shown, are identical to that of B wStr. The B wNo sequence has eight alignment gaps and nine unique residues.
HVR3 amino acids
In HVR3, five of 52 residues (Fig. 5, residues 224–277) are conserved among all strains. Throughout HVR3, sequences from the upper cluster of four A-strains are identical, including an alignment gap. However, the A wAna sequence has 22 unique residues (in green) and is partially conserved with B wNo (nine residues in orange). In striking contrast to differences in HVR1 and HVR2, the A wCobU4-2 and U5-2 homologs have identical HVR3 sequences that are conserved with the B-strains, particularly B wStr (residues in blue), differing only at residues 241 and 244.
Nucleotide sequence alignment confirms a mosaic wspB and identifies a conserved repeated sequence
Nucleotide sequence alignment of eleven wspB homologs confirmed that WOL-A/B genetic mosaicism is concentrated in the HVR regions and revealed three copies of a repeated sequence element within or near HVR2. Further analyses identified three copies of the repeated sequence element in ribA at the 5′-end of the virB8-D4 operon and four copies in vir genes.
HVR1
HVR1 (Fig. S2, nucleotides 117–241) from B wStr begins with two nucleotides (117 and 120 in red) that are conserved in B wStr and all WOL-A-strains except A wCobU5-2 and A wCobU4-2. Downstream, the B wStr sequence includes 47 of 48 nucleotides (in blue) within a sequence motif characteristic of B wStr and the other B-strains. The A wCobU5-2 and A wAna sequences are initially similar to the WOL-B motif, but beginning at an alignment gap in the other A-strains they have 11 nucleotides (in orange, nucleotides 152–207) that are conserved with B wNo and B wPip at positions in which those strains diverge from the WOL-B consensus. Thus, HVR1 in B wStr begins with nucleotides from a conserved WOL-A sequence motif but transitions to the conserved WOL-B motif, while HVR1 from the A wCobU4-2 A-strain differs from that WOL-B motif at a single nucleotide (176). In contrast, the A wAna and A wCobU5-2 sequences are mosaics of the WOL-A and WOL-B consensus motifs and share nucleotides with the divergent B wNo and B wPip B-strains, which also closely resemble each other upstream of HVR1 (23 nucleotides in light blue and one in orange).
HVR2 contains conserved repeat elements
HVR2 (Fig. S2, nucleotides 361–450) contains a conserved WOL-B sequence motif that differs at 20 nucleotides (in blue), from the WOL-A motif, while the divergent sequences from B wNo, B wPip, A wAna and A wCobU5-2 share an alignment gap and are again similar (nucleotides in orange). A tandem repeated sequence at nucleotides 365–379, CAAGTAA T CAAGTAAC, in the B-strains B wStr, B wVitB and B wMet occurs with slight variation (underlined residues) as CAAGTA G CCAA A TAAC, in the A-strains A wAtab 3, A wKue, A wMel and A wRi. We designated the eight-bp sequence, CAARTARY, where R = A or G, and Y = C or T, as an HVR2-repeat. The pseudogene from A wCobU4-2 contained a third copy of CAAGTAAT that interrupted ORF1 and was removed from the alignment (indicated by upwards arrow below alignment) to shift to ORF3, which maintains identity to the deduced amino acid sequence from B wStr. Just downstream of HVR2 at nucleotides 457–463, a truncated copy of the HVR2-repeat lacking the 3′-terminal pyrimidine is conserved in B wStr, B wVitB, B wMet and A wCobU4-2 and corresponds to the position (indicated by greater than less than symbols below alignment) of the transposon insertion in B wPip. Finally, we noted that the most divergent HVR2 sequences from A wAna, A wCobU5-2, B wNo and B wPip have T/C and A/G substitutions (in orange, light blue and green) that disrupt the HVR2-repeat consensus.
HVR3
Within HVR3 (Fig. S2, nucleotides 670–831), conserved sequence motifs occur in the upper cluster of four A-strains and in the B-strains (nucleotides in blue), with the exceptions of B wPip (HVR3 absent) and B wNo. Sequences from A wCobU4-2 and A wCobU5-2 are identical despite their major differences in HVR1 and HVR2 and differ from the B-strain consensus only at nucleotides 722 and 773 (in orange). The A wAna and B wNo sequences are the most divergent but share 43 variable nucleotides (in orange) and have 67 and 18 unique residues (in green), respectively.
HVR2-repeats also occur in ribA and ribB
Based on a DNA pattern search (http://bioinformatics.org/sms/), three HVR2-repeats occur in ribA, two in virD4, and single copies in virB8 and virB9 (Table 3). In addition, a reverse complement of the CAARTARY sequence occurs at the same position in ribB from three WOL-A-strains and B wPip (see gray shading in Fig. S3). The B wPip homolog contains a second copy at residues 7–14 just downstream of the start codon (not shown) and is a WOL-A/B mosaic (see below). Although repeat frequencies in individual ribA (0.29) and wspB (0.34) genes are ~sixfold higher than in the whole genomes of A wMel and B wPip (0.05) from flies (Diptera), it will be important to re-evaluate these frequencies when a B wStr genome (Hemipteran host) becomes available.
Although RibA and RibB are involved in riboflavin biosynthesis, ribB is not contiguous with ribA and the virV8-D4 operon, and it has higher variability than ribA (Table 2). Among the WOL-B-strains, ribB in B wStr and B wNo is conserved with the A wAu and A wMel A-strains (Fig. S3; note especially the bold blue residues downstream of nucleotide 181, as well as additional residues in orange). In contrast, the B wPip homolog is best-conserved (nucleotides in red) with WOL-A-strains, A wAna, A wHa and A wRi, including an alignment gap at residue 483 encompassing an identical 15-nucleotide “island” with the reverse complement CAARTARY repeat. Downstream of the gap, at residue 511, the B wPip sequence shifts to a predominantly WOL-B motif conserved in B wStr, B wNo, but also in A wMel (nucleotides in blue), while A wAna, A wRi and A wHa are mutually conserved (nucleotides in orange) versus all other strains. Within the 3′-end of the alignment (nucleotides 541–600), the B wPip sequence is conserved with B wStr, B wNo and D wBm (nucleotides in blue), while A wAu and A wMel are the most divergent (nucleotides in green).
Discussion
Although the status of Wolbachia as a species remains unclear (Baldo et al. 2006b; Lo et al. 2007), a notable distinction between WOL-C-/D-strains that associate with nematodes as mutualists and WOL-A-/B-strains that occur as reproductive parasites in insects relates to genome stability and phylogenetic congruence between Wolbachia and its host. In insect hosts, Wolbachia appears to engage in frequent horizontal gene transfer, resulting in a lack of phylogenetic congruence manifested by gene structures that represent mosaic recombinations from genomes now considered distinct strains. Coinfections with two or more Wolbachia strains and activities of bacteriophages that reside in genomes of WOL-A/B-strains likely contribute to this genetic plasticity (Bordenstein and Reznikoff 2005; Newton and Bordenstein 2011), which may reflect what some authors suggest is a worldwide Wolbachia pandemic (Zug et al. 2012). Examples of natural coinfections include A wAlbA and B wAlbB in A. albopictus mosquitoes (O’Neill et al. 1997), A wVitA and B wVitB in the parasitoid wasp, N. vitripennis (Perrot-Minnot et al. 1996; Raychoudhury et al. 2008) and A wHa and B wNo in the phytophagous D. simulans (James et al. 2002). A particularly interesting example in C. obstrictus weevils involves infection with a single A wCob strain, in which polymorphisms in wspA and wspB indicate that three distinct variants coexist in all host populations (Floate et al. 2011) and it will be of interest to explore other genetic similarities and differences among these variants following separation in vitro and/or in uninfected hosts. Wolbachia coinfections have also been documented in insects such as fig wasps (Yang et al. 2012), tephritid flies (Morrow et al. 2014) and planthoppers (Zhang et al. 2013) whose interactions with parasitoids, parasites and predator arthropods may facilitate horizontal transmission (Cordaux et al. 2001; Werren et al. 2008; Zug et al. 2012). In nature, the B wStr strain occurs in two planthopper hosts (Noda et al. 2001a) and in the strepsipteran endoparasite Elenchus japonicus (Noda et al. 2001b; Zhang et al. 2013). In the present study, B wStr has been artificially introduced into a cultured cell line, which has not been achieved with B wPip or nematode-associated strains. Adaptation of B wStr to cell lines (Noda et al. 2002; Fallon et al 2013) will provide an in vitro system for examining mechanisms of genetic exchange if conditions for maintenance of doubly infected cells can be developed through coinfection or somatic cell fusion. We note that high rates of recombination and transposition in Wolbachia (Baldo et al. 2006a; Cordaux et al. 2008) are consistent with expression of an abundant RecA protein (SR 1.05; Table S3, entry 146) as well as 18 transposases and/or proteins with transposase domains in B wStr (Baldridge et al. 2014).
Genetic plasticity of wspB in the virB8-D4 operon
An intact wspB that maps to the 3′-end of the virB8-D4 operon in most WOL-A genomes (Wu et al. 2004) is absent from 17 of 21 WOL-B-strains, including B wVulC and nearly all other isopod-associated strains (Pichon et al. 2009), and is interrupted by a transposon in B wPip (Sanogo et al. 2007). Here, we verify that in B wStr, an intact wspB is co-transcribed with virD4 and is expressed in C/wStr1 cells as an abundant protein at levels similar to those of many housekeeping proteins. The wspB structure closely resembles that of its better-studied wspA paralog, encoding a major surface antigen that has four HVR regions with sequence motifs that have been shuffled by recombination within and between Wolbachia WOL-A- and -B-strains (Baldo et al. 2005, 2010). Likewise, most sequence variation in wspB alleles occurs in the three HVR regions, with distinctive patterns for each region. HVR1 underscores WOL-A/B mosaicism in A wAna and A wCobU5-2, and in addition it shows a high level of identity between A wCobU4-2 and B wStr. Similarity between A wAna and A wCobU5-2 and between B wStr and A wCobU4-2 also occurs in HRV2, while B wNo stands out as distinctive. In B wPip, HVR2 is disrupted by a transposon insertion and we identified an eight-nucleotide HRV2-repeat (CAARTARY) that correlates with transitions between WOL-A-/B-strain motifs and the pseudogene lesions in B wPip and A wCobU4-2. Finally, we noted that high identity of A wCobU5-2, A wCobU4-2 and B wStr is unique to HVR3.
The remarkable similarity of the wspB homologs from B wStr and A wCobU4-2 (>98 % nucleotide identity Fig. S2) is consistent with exchange of an apparently intact gene between members of distinct Wolbachia supergroups by a mechanism that requires further investigation. Intensive analysis of the wspA paralog demonstrates that intragenic recombination breakpoints are concentrated in conserved regions outside of the HVRs (Baldo et al. 2005, 2010). CAARTARY repeats are not present in wspA, and in wspB, they occur only within and directly adjacent to HVR2 at positions that correspond to pseudogene lesions in A wCobU4-2 and in B wPip (due to a transposition event in B wPip; Sanogo et al. 2007). Furthermore, Pichon et al. (2009) suggested that transposition events may explain absence of wspB in the virB8-D4 operons of many WOL-B-strains. In a practical sense, CAARTARY repeats at wspB pseudogene lesions and WOL-A/B sequence motif transitions (Figs. S1, S2, S3) suggest their involvement in genetic exchange. Because transformation of Wolbachia has not yet been achieved, engineering of CAARTARY repeats into vectors used successfully to introduce selectable markers into other members of the Rickettsiales (see Beare et al. 2011) merits investigation.
Potential functions of WspB
Although bacterial outer membrane proteins are important mediators of interactions with host cells and specific function(s) of both WspA and WspB remain to be identified, they may have unique functions as porin proteins in Wolbachia, which lack cell walls. The virB8-D4 operons of Wolbachia and its sister genera, Anaplasma and Ehrlichia, are similarly organized (Gillespie et al. 2010; Hotopp et al. 2006) with 3′- terminal genes encoding major surface proteins that, analogous to wspB, are co-transcribed with the vir genes (Ohashi et al. 2002). In A. marginale, a family of msp2 pseudogenes undergo “combinatorial gene conversion” at the expression site (Brayton et al. 2002) and MSP2 variants change during growth in different host cell types, which likely reflects a response to host immunity mechanisms (Chávez et al. 2012). Similarly, Baldo et al. (2010) proposed that changes in WspA HVR regions play a role in host adaptation and innate immunity interactions, consistent with variation in the higher-order structure of the protein in different hosts (Uday and Puttaraju 2012). HVR sequence changes in the wspB paralog may reflect a similar dynamic. Additional evidence indicates that MSP2 proteins are glycosylated (Sarkar et al. 2008), which is now an established process in post-translational modification in bacteria (Dell et al. 2010; Nothaft and Szymanski 2010), and we note that WspB contains potential glycosylation sites. Although an inactivated pseudogene or absence of wspB in virB8-D4 operons of some Wolbachia strains indicates that it is not absolutely required for survival, a secretome analysis of Brugia malayi showed that WspB from D wBm is excreted/secreted into filarial host cells (Bennuru et al. 2009). Furthermore, it co-localizes with the Bm1_46455 host protein in tissues that include embryonic nuclei (Melnikow et al. 2011). WspB is therefore itself a candidate T4SS effector that may play a role in reproductive manipulation of the host. Mosaicism in wspB and its high rate of evolution (Comandatore et al. 2013) may thus reflect genetic changes that optimize adaptation to particular host cells such as those in reproductive tissues and facilitate exploitation of new arthropod niches by Wolbachia.
Genetic plasticity of ribA in the virB8-D4 operon
Aside from wspB at the 3′-end of the T4SS virB8-D4 operon, ribA exhibits genetic plasticity at its 5′-end. In both B wStr and B wVulC, ribA is a two-part mosaic of N-terminal WOL-A and C-terminal WOL-B motifs. In contrast, the internal virB8-D4 genes have typical B-strain identities, and in some strain comparisons, amino acid identities slightly exceed nucleotide identities, which Pichon et al. (2009) attribute to strong selection against non-synonymous codon substitutions. Among the internal virB8-D4 genes, however, Klasson et al. (2009) suggest that in A wRi, an especially variable region in virB10 is likely derived from genetic exchange with a B-strain. We note here that ribA from A wRi closely resembles B-strain homologs within a variable region that immediately precedes the GTP cyclohydrolase domain, where its homolog in B wStr transitions from WOL-A to WOL-B sequence motifs (Fig. S1, positions 387–450).
In contrast to D wBm, in which ribA and virB8 are co-transcribed and bind common transcription factors (Li and Carlow 2012), relative abundance levels suggest that in B wStr, ribA is transcribed independently of the virB8-D4 operon. Some WOL-B-strains, such as B wVulC, lack wspB at the 3′-terminus of the virB8-D4 operon, while our data confirm that in B wStr, wspB is co-transcribed with the vir genes, consistent with similar relative abundances of WspB and the five Vir proteins. In aggregate, these observations suggest that WOL-D and WOL-A-/B-strains may differ in how RibA and WspB expression interfaces with T4SS-mediated transport of effectors in filarial worms and arthropod hosts (Felix et al. 2008; Masui et al. 2000; Rances et al. 2008; Wu et al. 2004), and it will be of interest to explore whether such differences relate to riboflavin provisioning. In filarial nematodes (Li and Carlow 2012; Strubing et al. 2010; Wu et al. 2009) and bedbugs (Hosokawa et al. 2010), evidence suggests that Wolbachia provisions host with riboflavin, the precursor of flavin cofactors that are essential for many cellular redox reactions. In contrast, riboflavin depletion reduces B wStr abundance in C/wStr1 cells, suggesting that B wStr utilizes host riboflavin and does not augment riboflavin levels in mosquito host cells (Fallon et al. 2014).
Potential functions of RibA and RibB
In initial commitment steps in riboflavin biosynthesis, enzymatic activities encoded by the ribA and ribB functional domains use GTP and ribulose-5-phosphate as substrates to catalyze riboflavin biosynthesis, consuming 25 molecules of ATP per molecule of riboflavin (Bacher et al. 2000). We note that in Wolbachia genomes, ribA is the annotated homolog of ribBA in Escherichia coli (Brutinel et al. 2013) and encodes a dihydroxybutanone phosphate synthase domain with putative RibB function near the N-terminus, upstream of a GTP cyclohydrolase II domain with conserved dimerization and active site residues (RibA function). As in E. coli, Wolbachia genomes also encode ribB, but at a distinct chromosomal locus, suggesting that ribA and ribB are not coordinately expressed. In Sinorhizobium meliloti (Rhizobiales; Alphaproteobacteria), knockout mutations of ribBA decreased flavin secretion but did not cause riboflavin auxotrophy or block establishment of symbiosis, suggesting that RibBA may have an undefined role in molecular transport (Yurgel et al. 2014). As is the case with B wStr, RibB is at least threefold more abundant than RibA in the bacterium Acidithiobacillus ferrooxidans (Knegt et al. 2008). In yeast, RibB has thiol-dependent alternative redox states (McDonagh et al. 2011), partially localizes to the mitochondrial periplasm, and has an unexplained function in oxidative respiration that is independent of riboflavin biosynthesis (Jin et al. 2003). These observations raise the possibility that in Wolbachia, RibA and RibB may have functions other than riboflavin biosynthesis that integrate with pathways involved in cellular oxidative state, such as iron metabolism. Intracellular bacteria are challenged by host-imposed oxidative stress and iron starvation (reviewed by Benjamin et al. 2010) and riboflavin biosynthesis is associated with iron acquisition in bacteria such as Helicobacter pylori (Worst et al. 1998) and Campylobacter jejuni (Crossley et al. 2007). Wolbachia interferes with iron metabolism and sequestration in insects (Brownlie et al. 2009; Kremer et al. 2009) and influences iron-dependent host processes such as heme metabolism, oxidative stress, apoptosis and autophagy (Gill et al. 2014). We note that the periplasmic iron-binding component of a membrane transporter is an abundant protein in B wStr (Table S3, entry 778 and Baldridge et al. 2014).
References
Alvarez-Martinez CE, Christie PJ (2009) Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev 73:775–808
Bacher A, Eberhardt S, Fischer M, Kis K, Richter G (2000) Biosynthesis of vitamin B2 (riboflavin). Annu Rev Nutr 20:153–167
Baldo L, Lo N, Werren JH (2005) Mosaic nature of the Wolbachia surface protein. J Bacteriol 187:5406–5418. doi:10.1128/JB.187.15.5406-5418.2005
Baldo L, Bordenstein S, Werengreen JJ, Werren JH (2006a) Widespread recombination throughout Wolbachia genomes. Mol Biol Evol 23:437–449
Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MC, Tettelin H, Werren JH (2006b) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110
Baldo L, Desjardins CA, Russell JA, Stahlhut JK, Werren JH (2010) Accelerated microevolution in an outer membrane protein (OMP) of the intracellular bacteria Wolbachia. BMC Evol Biol. doi:10.1186/1471-2148-10-48
Baldridge GD, Baldridge AS, Witthuhn BA, Higgins L, Markowski TW, Fallon AM (2014) Proteomic profiling of a robust Wolbachia infection in an Aedes albopictus mosquito cell line. Mol Microbiol 94:537–556. doi:10.1111/mmi.12768
Beare PA, Sandoz KM, Omsland A, Rockey DD, Heinzen RA (2011) Advances in genetic manipulation of obligate intracellular bacteria. Front Microbiol 2:97. doi:10.3389/fmicb.2011.00097
Benjamin JA, Desnoyers G, Morissette A, Salvail H, Massé E (2010) Dealing with oxidative stress and iron starvation in microorganisms: an overview. Can J Physiol Pharmacol 88:264–272
Bennuru S, Semnani R, Meng Z, Ribeiro JMC, Veenstra TD, Nutman TB (2009) Brugia malayi excreted/secreted proteins at the host/parasite interface: stage-and gender-specific proteomic profiling. PLoS Negl Trop Dis 3:e410. doi:10.1371/journal.pntd.0000410
Bigelow HR, Petrey DS, Liu J, Przybylski D, Rost B (2004) Predicting transmembrane beta-barrels in proteomes. Nucleic Acids Res 32:2566–2577
Bordenstein SR, Reznikoff WS (2005) Mobile DNA in obligate intracellular bacteria. Nat Rev Microbiol 3:688–699
Bourtzis K (2008) Wolbachia-based technologies for insect pest population control. Adv Exp Med Biol 627:104–113. doi:10.1007/978-0-387-78225-6_9
Brayton KA, Palmer GH, Lundgren A, Yi J, Barbet AF (2002) Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol Microbiol 43:1151–1159
Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL (2009) Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5:e1000368. doi:10.1371/journal.ppat.1000368
Brutinel ED, Dean AM, Gralnick JA (2013) Description of a riboflavin biosynthetic gene variant prevalent in the phylum proteobacteria. J Bacteriol 195:5479–5486
Chávez AS, Felsheim RF, Kurtti TJ, Ku PS, Brayton KA, Munderloh UG (2012) Expression patterns of Anaplasma marginale Msp2 variants change in response to growth in cattle, and tick cells versus mammalian cells. PLoS One 7(4):e36012. doi:10.1371/journal.pone.0036012
Comandatore F, Sassera D, Montagna M, Kumar S, Darby A, Blaxter M et al (2013) Phylogenomics and analysis of shared genes suggest a single transition to mutualism in Wolbachia of nematodes. Genome Biol Evol 5:1668–1674
Cordaux R, Michel-Salzat A, Bouchon D (2001) Wolbachia infection in crustaceans: novel hosts and potential routes for horizontal transmission. J Evol Biol 14:237–243
Cordaux R, Pichon S, Ling A, Perez P, Delaunay C, Vavre F, Bouchon D, Greve P (2008) Intense transpositional activity of insertion sequences in an ancient endosymbiont. Mol Biol Evol 25:1889–1895. doi:10.1093/molbev/msn134
Crossley RA, Gaskin DJ, Holmes K, Mulholland F, Wells JM, Kelly DJ, van Vliet AH, Walton NJ (2007) Riboflavin biosynthesis is associated with assimilatory ferric reduction and iron acquisition by Campylobacter jejuni. Appl Environ Microbiol 73:7819–7825
Dedeine F, Bandi C, Bouletreau M, Kramer L (2003) Insights into Wolbachia obligatory symbiosis. In: Bourtzis K, Miller TA (eds) Insect symbiosis. CRC Press, Florida, pp 267–282
Dell A, Galadari A, Sastre F, Hitchen P (2010) Similarities and differences in the glycosylation mechanisms in prokaryotes and eukaryotes. Int J Microbiol. doi:10.1155/2010/148178
Fallon AM (2008) Cytological properties of an Aedes albopictus mosquito cell line infected with Wolbachia strain wAlbB. In Vitro Cell Dev Biol Anim 44:154–161. doi:10.1007/s11626-008-9090-4
Fallon AM, Baldridge GD, Higgins LA, Witthuhn BA (2013) Wolbachia from the planthopper Laodelphax striatellus establishes a robust, persistent, streptomycin-resistant infection in clonal mosquito cells. In Vitro Cell Dev Biol Anim 49:66–73. doi:10.1007/s11626-012-9571-3
Fallon AM, Baldridge GD, Carroll EM, Kurtz CM (2014) Depletion of host cell riboflavin reduces Wolbachia in cultured mosquito cells. In Vitro Cell Dev Biol Anim 50:707–713. doi:10.1007/s11626-014-9758-x
Felix C, Pichon S, Braquart-Varnier C, Braig H, Chen L, Garrett RA, Martin G, Greve P (2008) Characterization and transcriptional analysis of two gene clusters for type IV secretion machinery in Wolbachia of Armadillidium vulgare. Res Microbiol 159:481–485
Floate KD, Coghlin PC, Dosdall L (2011) A test using Wolbachia bacteria to identify Eurasian source populations of Cabbage Seed Pod Weevil, Ceutorhynchus obstrictus (Marsham), in North America. Environ Entomol 40:818–2011. doi:10.1603/EN10315
Gill AG, Darby AC, Makepeace BL (2014) Iron necessity: the secret of Wolbachia’s success? PLoS Negl Trop Dis 16:e3224. doi:10.1371/journal.pntd.0003224
Gillespie JJ, Ammerman NC, Dreher-Lesnick SM, Rahman MS, Worley MJ, Setubal JC, Sobral BS, Azad AF (2009) An anomalous type IV secretion system in Rickettsia is evolutionarily conserved. PLoS One 4:e4833. doi:10.1371/journal.pone.0004833
Gillespie JJ, Brayton KA, Williams KP, Quevado Diaz MA, Brown WC, Azad AF, Sobral BW (2010) Phylogenomics reveals a diverse Rickettsiales type IV secretion system. Infect Immun 78:1809–1823
Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia? A statistical analysis of current data. FEMS Microbiol Lett 281:215–220
Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T (2010) Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA 107:769–774
Hotopp JC, Lin M, Madupu R, Crabtree SV, Angiloui SV et al (2006) Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet 2:e21
James AC, Dean MD, McMahon ME, Ballard JW (2002) Dynamics of double and single Wolbachia infections in Drosophila simulans from New Caledonia. Heredity 88:182–189
Jin C, Barrientos A, Tzagoloff A (2003) Yeast dihydroxybutanone phosphate synthase, an enzyme of the riboflavin biosynthetic pathway, has a second unrelated function in expression of mitochondrial respiration. J Biol Chem 278:14698–14703
Kambris Z, Cook PE, Phuc HK, Sinkins SP (2009) Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326:134–136. doi:10.1126/science.1177531
Klasson L, Westberg J, Sapountzis P, Naslund K, Lutnaes Y, Darby AC, Veneti Z, Chen L, Braig HR, Garret R et al (2009) The mosaic structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc Natl Acad Sci USA 106:5725–5730
Knegt FH, Mello LV, Reis FC, Santos MT, Vicentini R, Ferraz LF, Ottoboni LM (2008) ribB and ribBA genes from Acidithiobacillus ferrooxidans: expression levels under different growth conditions and phylogenetic analysis. Res Microbiol 159:423–431
Koebnik R, Locher KP, Van Gelder P (2000) Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol Microbiol 37:239–253
Kremer N, Voronin D, Charif D, Mavingui P, Mollereau B, Vavre F (2009) Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog 5:e1000630. doi:10.1371/journal.ppat.1000630
Li Z, Carlow CKS (2012) Characterization of transcription factors that regulate the type IV secretion system and riboflavin biosynthesis in Wolbachia of Brugia malayi. PLoS One 7:e51597. doi:10.1371/journal.pone0051597
Liu H, Bao W, Lin M, Niu H, Rikihisa Y (2012) Ehrlichia type IV secretion effector ECH0825 is translocated to mitochondria and curbs ROS and apoptosis by upregulating MnSOD. Cell Microbiol 14:1037–1050. doi:10.1111/j.1462-5822.2012.01775.x
Lo N, Paraskevopoulos C, Bourtzis K, O’Neill SL, Werren JH, Bordenstein SR, Bandi C (2007) Taxonomic status of the intracellular bacterium Wolbachia pipientis. Int J Syst Evol Microbiol 57:654–657
Lockwood S, Voth DE, Brayton KA, Beare PA, Brown WC, Heinzen RA, Broschat SL (2011) Identification of Anaplasma marginale type IV secretion system effector proteins. PLoS One 6:e27724. doi:10.1371/journal.pone.0027724
Masui S, Sasaki T, Ishikawa H (2000) Genes for the type IV secretion system in an intracellular symbiont, Wolbachia, a causative agent of various sexual alterations in arthropods. J Bacteriol 182:6529–6531
McDonagh B, Reguejo R, Fuentes-Almagro CA, Ogueta S, Bárcena JA, Padilla CA (2011) Thiol redox proteomics identifies differential targets of cytosolic and mitochondrial glutaredoxin-2 isoforms in Saccharomyces cerevisiae. Reversible S-glutathionylation of DHBP synthase (RIB3). J Proteomics 74:2487–2497
Melnikow E, Xu S, Liu J, Li L, Oksov Y, Ghedin E et al (2011) Interaction of a Wolbachia WSP-like protein with a nuclear-encoded protein of Brugia malayi. Int J Parasitol 41:1053–1061
Morrow JL, Frommer M, Shearman DC, Riegler M (2014) Tropical tephritid fruit fly community with high incidence of shared Wolbachia strains as platform for horizontal transmission of endosymbionts. Environ Microbiol 16:3622–3637. doi:10.1111/1462-2920.12382
Newton LG, Bordenstein SR (2011) Correlations between bacterial ecology and mobile DNA. Curr Microbiol 62:198–208. doi:10.1007/s00284-010-9693-3
Niu H, Kozjak-Pavlovic V, Rudel T, Rikihisa Y (2010) Anaplasma phagocytophilum Ats-1 is imported into host cell mitochondria and interferes with apoptosis induction. PLoS Pathog 6:e1000774
Noda H, Koizumi Y, Zhang Q, Deng K (2001a) Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem Mol Biol 31:727–737
Noda H, Miyoshi T, Zhang Q, Watanabe K, Deng K, Hoshizaki S (2001b) Wolbachia infection shared among planthoppers (Homoptera: Delphacidae) and their endoparasite (Strepsiptera: Elenchidae): a probable case of interspecies transmission. Mol Ecol 10:2101–2106
Noda H, Myoshi T, Koizumi Y (2002) In vitro cultivation of Wolbachia in insect and mammalian cell lines. In Vitro Cell Dev Biol Anim 38:423–427
Nothaft H, Szymanski CM (2010) Protein glycosylation in bacteria: sweeter than ever. Nat Rev 8:775–778. doi:10.1038/nrmicro2383
Ofran Y, Rost B (2006) ISIS: interaction sites identified from sequence. Bioinformatics 23:e13–e16. doi:10.1093/bioinformatics/bt303
Ohashi N, Zhi N, Lin Q, Rikihisa Y (2002) Characterization and transcriptional analysis of gene clusters for a type IV secretion machinery in human granulocytic and monocytic ehrlichiosis agents. Infect Immun 70:2128–2138
O’Neill SL, Pettigrew MM, Sinkins SP, Braig HR, Andreadis TG, Tesh RB (1997) In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol 6:33–39
Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z (2012) Wolbachia induces reactive oxygen species (ROS)-dependent activation of the toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA 109:E23–E31. doi:10.1073/pnas.1116932108
Perrot-Minnot MJ, Guo LR, Werren JH (1996) Single and double infections with Wolbachia in the parasitic wasp Nasonia vitripennis: effects on compatibility. Genetics 143:961–972
Pichon S, Bouchon D, Cordaux R, Chen L, Garret RA, Greve P (2009) Conservation of the type IV secretion system throughout Wolbachia evolution. Biochem Biophys Res Commun 385:557–562
Rances E, Voronin D, Tran-Van V, Mavangui P (2008) Genetic and functional characterization of the type IV secretion system in Wolbachia. J Bacteriol 190:5020–5030
Raychoudhury R, Baldo L, Oliveira DCSG, Werren JH (2008) Modes of acquisition of Wolbachia: horizontal transfer, hybrid introgression, and convergence in the Nasonia species complex. Evolution 63:165–183. doi:10.1111/j.1558-5646.2008.00533.x
Rio R, Hu Y, Aksoy S (2004) Strategies of the home-team: symbioses exploited for vector-borne disease control. Trends Microbiol 12:325–336. doi:10.1016/j.tim.2004.05.001
Sanogo YO, Dobson SL, Bordenstein SR, Novak RJ (2007) Disruption of the Wolbachia surface protein gene wspB by a transposable element in mosquitoes of the Culex pipiens complex (Diptera, Culicidae). Insect Mol Biol 16:143–154
Saridaki A, Bourtzis K (2010) Wolbachia: more than just a bug in insect genitals. Curr Opin Microbiol 13:67–72
Sarkar M, Troese MJ, Kearns SA, Yang T, Reneer DV, Carlyon JA (2008) Anaplasma phagocytophilum MSP2(P44)-18 predominates and is modified into multiple isoforms in human myeloid cells. Infect Immun 76:2090–2098. doi:10.1128/IAI.01594-07
Schuler H, Bertheau C, Egan SP, Feder JL, Riegler M, Schlick-Steiner BC, Steiner FM, Johannesen J, Kern P, Tuba K, Lakatos F, Koppler K, Arthofer W, Stauffer C (2013) Evidence for a recent horizontal transmission and spatial spread of Wolbachia from endemic Rhagoletis cerasi (Diptera: Tephritidae) to invasive Rhagoletis cingulata in Europe. Mol Ecol 22:4101–4111. doi:10.1111/mec.12362
Shih KM, Gerenday A, Fallon AM (1998) Culture of mosquito cells in Eagle’s medium. In Vitro Cell Dev Biol Anim 34:629–630
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Sodung J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi:10.1038/msb.2011.75
Sinkins SP, Gould F (2006) Gene drive systems for insect disease vectors. Nat Rev Genet 7:427–435
Strubing U, Lucius R, Hoerauf A, Pfarr KM (2010) Mitochondrial genes for heme-dependent respiratory chain complexes are up-regulated after depletion of Wolbachia from filarial nematodes. Int J Parasitol 15:1193–1202. doi:10.1016/j.ijpara.2010.03.004
Swofford DL (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland
Taylor MJ, Bandi C, Hoerauf A (2005) Wolbachia bacterial endosymbionts of filarial nematodes. Adv Parasitol 60:245–284
Uday J, Puttaraju HP (2012) Comparative analysis of Wolbachia surface protein in D. melanoagster, A. tabida and B. malayi. Bioinformation 8(15):711–715. doi:10.6026/97320630008711
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751
Worst DJ, Gerrits MM, Vandenbroucke-Grauls CM, Kusters JG (1998) Helicobacter pylori ribBA-mediated riboflavin production is involved in iron acquisition. J Bacteriol 180:1473–1479
Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw E, Martin W, Esser C et al (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2:0327–0341
Wu B, Novelli J, Foster J, Vaisvila R, Conway L, Ingram J, Ganatra M, Rao AU, Hamza I, Slatko B (2009) The heme biosynthetic pathway of the obligate Wolbachia endosymbiont of Brugia malayi as a potential anti-filarial drug target. PLoS Negl Trop Dis 3:e475. doi:10.1371/journal.pntd.0000475
Yang C-H, Xiao J-H, Niu L-M, Ma G-C, Cook JM, Bian S-N, Fu Y-G, Huang D-H (2012) Chaos of Wolbachia sequences inside the compact fig syconia of Ficus benjamina (Ficus: Moraceae). PLoS One. doi:10.1371/journal.pone.0048882
Yurgel SN, Rice J, Domreis E, Lynch J, Sa N, Qamar Z, Rajamani S, Gao M, Roje S, Bauer WD (2014) Sinorhizobium meliloti flavin secretion and bacteria-host interaction: role of the bifunctional RibBA protein. Mol Plant-Microbe Interact 27:437–445. doi:10.1094/MPMI-11-13-0338-R
Zabalou S, Riegler M, Theodorakopoulou M, Stauffer C, Savakis C, Bourtzis K (2004) Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc Natl Acad Sci USA 101:15042–15045. doi:10.1073/pnas.0403853101
Zechner EL, Lang S, Schildbach JF (2012) Assembly and mechanisms of bacterial type IV secretion machines. Phil Trans R Soc B 367:1073–1087. doi:10.1098/rstb.2011.0207
Zhang KJ, Han X, Hong XY (2013) Various infection status and molecular evidence for horizontal transmission and recombination of Wolbachia and Cardinium among rice planthoppers and related species. Insect Sci 3:329–344. doi:10.1111/j.1744-7917.2012.01537.x
Zug R, Hammerstein P (2014) Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol Rev. doi:10.1111/brv.12098
Zug R, Koehncke A, Hammerstein P (2012) Epidemiology in evolutionary time: the case of Wolbachia horizontal transmission between arthropod species. J Evol Biol 25:2149–2160. doi:10.1111/j.1420-9101.2012.02601.x
Acknowledgments
This work was supported by Grant AI 081322 from the National Institutes of Health and by the University of Minnesota Agricultural Experiment Station, St. Paul, MN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Communicated by Markus Nett.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Polymerase chain reaction primers and amplification products obtained from the B wStr genes, ribA, ribB, virB8-D4, wspB and topA. (DOCX 108 kb)
Table S2
Genbank accession numbers for all Wolbachia homologs of ribA, ribB, virB8-D4, wspB and topA, including those from B wStr. N/A: not applicable either because the sequences are not available or were not used in the comparisons reported in the tables and figures. The C wOv genome is not annotated and the numerical values refer to genome coordinates determined by BLAST comparisons to B wStr. (XLSX 177 kb)
Table S3
Results of univariable and multivariable analyses after log transformation of the outcome, Peptide Count, and predictor, Molecular Weight. This table reports results for the refined search of the MS data sets with inclusion of sequences of cloned B wStr genes reported here and highlighted in yellow within the Table. Results of the original search of the four MS data sets were detailed previously (Baldridge et al. 2014). See tabs at bottom: Sheet 1 reports Mean SR values for all proteins in original and refined models in columns M and R; Univariable model and Multivariable Model (adjusted for functional class and MS Dataset) for results of tests of association. Runs 1, 2, 3 and 4 correspond to MS data sets D, E, F and G, respectively. (XLS 283 kb)
Figure S1
Nucleotide alignment of ribA homologs from B wStr and WOL-A, B- and D-strains at left in red, blue and black font, respectively. Nucleotides encoding the dihydroxybutanone phosphate synthase and GTP cyclohydrolase II domains are indicated above the alignment within greater than less than symbols. Asterisks below alignment indicate universally conserved nucleotides. Unique nucleotides are in green font. Nucleotides conserved in B wStr and a majority of B-strains are in dark blue bold font, while those in dark red bold font are conserved with a majority of A-strains. Nucleotides conserved in two to four strains are in light blue, orange or orange bold font. Nucleotides highlighted in gray and cyan indicate the MS-detected A wMel and B wPip 95% confidence peptides shown in Fig. 1, with amino acids indicated at top. Underlined nucleotides correspond to the CAARTARY repeat. See Tables 2 and S2 for host associations and Genbank Accessions. (DOCX 292 kb)
Figure S2
Nucleotide sequence alignment of wspB homologs from B wStr and WOL-A and B-strains as indicated by red and blue font at left. Nucleotides conserved between B wStr and a majority of A-strains are in red font and residues conserved with a majority of B-strains are in blue font. Asterisks below alignment indicate universally conserved nucleotides and double underlines above the alignment indicate three hypervariable regions (HVRs). Unique nucleotides are in green font and residues conserved between two to four strains are in light blue, orange or bold orange font. The greater than less than symbols below alignment indicate a transposon insertion in the wspB pseudogene of B wPip, which is aligned as three discontinuous sequence blocks corresponding to nucleotides 1334165 - 1334594; 1335958 - 1336167; 1336271 – 1336326 from Accession NC_010981.1. The three CAARTARY repeats are underlined (nucleotides 365–379 and 457–463). Highlighted residues correspond to 95% confidence peptides detected by LC–MS/MS (amino acids indicated at top; lower case indicates additional matched peptides not unique to WspB) that were conserved in most strains (gray), conserved in B-strains (cyan), conserved in B wStr and A wCobU4-2 (yellow), or unique to B wStr (olive). See Tables 2 and S2 for host associations and Genbank Accessions. (DOCX 271 kb)
Figure S3
Nucleotide sequence alignment of ribB homologs from B wStr and WOL-A, B- and D-strains at left in red, blue and black font, respectively. Asterisks below the alignment indicate universally conserved nucleotides. Unique nucleotides are in green font. Nucleotides conserved in B wStr and a majority of B-strains are in dark blue bold font, while those in dark red bold font are conserved with a majority of A-strains. Nucleotides conserved in two to four strains are in light blue, orange or orange bold font. See Tables 2 and S2 for host associations and Genbank Accessions. (DOCX 250 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Baldridge, G.D., Li, Y.G., Witthuhn, B.A. et al. Mosaic composition of ribA and wspB genes flanking the virB8-D4 operon in the Wolbachia supergroup B-strain, wStr. Arch Microbiol 198, 53–69 (2016). https://doi.org/10.1007/s00203-015-1154-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-015-1154-8