Abstract
Summary
We have calculated the biological variation (BV) of different bone metabolism biomarkers on a large, well-described cohort of subjects. BV is important to calculate reference change value (or least significant change) which allows evaluating if the difference observed between two consecutive measurements in a patient is biologically significant or not.
Introduction
Within-subject (CVI) and between-subject (CVG) biological variation (BV) estimates are essential in determining both analytical performance specifications (APS) and reference change values (RCV). Previously published estimates of BV for bone metabolism biomarkers are generally not compliant with the most up-to-date quality criteria for BV studies. We calculated the BV and RCV for different bone metabolism markers, namely β-isomerized C-terminal telopeptide of type I collagen (β-CTX), N-terminal propeptide of type I collagen (PINP), osteocalcin (OC), intact fibroblast growth factor 23 (iFGF-23), and uncarboxylated-unphosphorylated Matrix-Gla Protein (uCuP-MGP) using samples from the European Biological Variation Study (EuBIVAS).
Methods
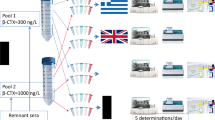
In the EuBIVAS, 91 subjects were recruited from six European laboratories. Fasting blood samples were obtained weekly for ten consecutive weeks. The samples were run in duplicate on IDS iSYS or DiaSorin Liaison instruments. The results were subjected to outlier and variance homogeneity analysis before CV-ANOVA was used to obtain the BV estimates.
Results
We found no effect of gender upon the CVI estimates. The following CVI estimates with 95% confidence intervals (95% CI) were obtained: β-CTX 15.1% (14.4–16.0%), PINP 8.8% (8.4–9.3%), OC 8.9% (8.5–9.4%), iFGF23 13.9% (13.2–14.7%), and uCuP-MGP 6.9% (6.1–7.3%).
Conclusions
The EuBIVAS has provided updated BV estimates for bone markers, including iFGF23, which have not been previously published, facilitating the improved follow-up of patients being treated for metabolic bone disease.




Similar content being viewed by others
References
Fraser CG (2001) The nature of biological variation. In: Biol. Var. From Princ. to Pract. AACC Press, Washington, pp 1–27
Fraser CG, Petersen PH (1999) Analytical performance characteristics should be judged against objective quality specifications. Clin Chem 45:321–323
Fraser CG (2009) Reference change values: the way forward in monitoring. Ann Clin Biochem 46:264–265. https://doi.org/10.1258/acb.2009.009006
Simundic A-M, Kackov S, Miler M et al (2015) Terms and symbols used in studies on biological variation: the need for harmonization. Clin Chem 61:436–438. https://doi.org/10.1373/clinchem.2014.232694
Perich C, Minchinela J, Ricós C, Fernández-Calle P, Alvarez V, Doménech MV, Simón M, Biosca C, Boned B, García-Lario JV, Cava F, Fernández-Fernández P, Fraser CG (2015) Biological variation database: structure and criteria used for generation and update. Clin Chem Lab Med 53:299–305. https://doi.org/10.1515/cclm-2014-0739
Aarsand AK, Røraas T, Fernandez-Calle P et al (2018) The biological variation data critical appraisal checklist: a standard for evaluating studies on biological variation. Clin Chem 64:501–514. https://doi.org/10.1373/clinchem.2017.281808
Carobene A (2015) Reliability of biological variation data available in an online database: need for improvement. Clin Chem Lab Med 53:871–877. https://doi.org/10.1515/cclm-2014-1133
Aarsand AK, Fernandez-Calle P, Webster C, et al. (2019) The EFLM biological variation database. In: https://biologicalvariation.eu
González-Lao E, Corte Z, Simón M et al (2019) Systematic review of the biological variation data for diabetes related analytes. Clin Chim Acta 488:61–67. https://doi.org/10.1016/j.cca.2018.10.031
Díaz-Garzón J, Fernández-Calle P, Minchinela J et al (2019) Biological variation data for lipid cardiovascular risk assessment biomarkers. A systematic review applying the biological variation data critical appraisal checklist (BIVAC). Clin Chim Acta 495:467–475. https://doi.org/10.1016/j.cca.2019.05.013
Alvarez L, Ricos C, Peris P et al (2000) Components of biological variation of biochemical markers of bone turnover in Paget’s bone disease. Bone 26:571–576
Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R (2002) Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 30:886–890. https://doi.org/10.1016/S8756-3282(02)00728-7
Bauer DC, Garnero P, Harrison SL et al (2009) Biochemical markers of bone turnover, hip bone loss, and fracture in older men: the MrOS study. J Bone Miner Res 24:2032–2038. https://doi.org/10.1359/JBMR.090526
Garnero P, Mulleman D, Munoz F et al (2003) Long-term variability of markers of bone turnover in postmenopausal women and implications for their clinical use: the OFELY study. J Bone Miner Res 18:1789–1794
Nguyen TV, Nelson AE, Howe CJ, Seibel MJ, Baxter RC, Handelsman DJ, Kazlauskas R, Ho KK (2008) Within-subject variability and analytic imprecision of insulin like growth factor axis and collagen markers: implications for clinical diagnosis and doping tests. Clin Chem 54:1268–1276. https://doi.org/10.1373/clinchem.2008.105726
Stevenson HP, Leslie H, Sheridan B (1997) Intra-individual variation in serum type I procollagen carboxy-terminal propeptide and type I collagen carboxy-terminal cross-linked telopeptide concentrations. Ann Clin Biochem 34:317–318. https://doi.org/10.1177/000456329703400316
Jensen JEB, Sørensen HA, Kollerup G et al (1994) Biological variation of biochemical bone markers. Scand J Clin Lab Invest 54:36–39. https://doi.org/10.3109/00365519409088575
Hannon R, Blumsohn A, Naylor KER (1998) Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variability. J Bone Miner Res 13:1124–1133
Panteghini M, Pagani F (1995) Biological variation in bone-derived biochemical markers in serum. Scand J Clin Lab Invest 55:609–616. https://doi.org/10.3109/00365519509110260
Jabor A, Kubíček Z, Komrsková J et al (2019) Biological variation of intact fibroblast growth factor 23 measured on a fully automated chemiluminescent platform. Ann Clin Biochem Int J Lab Med 0:000456321982616. https://doi.org/10.1177/0004563219826161
Carobene A, Strollo M, Jonker N, Barla G, Bartlett WA, Sandberg S, Sylte MS, Røraas T, Sølvik UØ, Fernandez-Calle P, Díaz-Garzón J, Tosato F, Plebani M, Coşkun A, Serteser M, Unsal I, Ceriotti F, Biological Variation Working Group, European Federation of Clinical Chemistry and Laboratory Medicine (2016) Sample collections from healthy volunteers for biological variation estimates’ update: a new project undertaken by the working group on biological variation established by the European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med 54:1599–1608. https://doi.org/10.1515/cclm-2016-0035
Carobene A (2017) The European Biological Variation Study (EuBIVAS): delivery of updated biological variation estimates, a project by the working group on biological variation in the European Federation of Clinical Chemistry and Laboratory Medicine. J Lab Precis Med 2:70–70. https://doi.org/10.21037/jlpm.2017.08.13
Carobene A, Røraas T, Sølvik UØ, Sylte MS, Sandberg S, Guerra E, Marino I, Jonker N, Barla G, Bartlett WA, Fernandez-Calle P, Díaz-Garzón J, Tosato F, Plebani M, Coşkun A, Serteser M, Unsal I, Ceriotti F, European Biological Variation Study of the EFLM Working Group on Biological Variation (2017) Biological variation estimates obtained from 91 healthy study participants for 9 enzymes in serum. Clin Chem 63:1141–1150. https://doi.org/10.1373/clinchem.2016.269811
Aarsand AK, Diaz-Garzon J, Fernandez-Calle P et al (2018) The EuBIVAS: within- and between-subject biological variation data for electrolytes, lipids, urea, uric acid, total protein, total bilirubin, direct bilirubin, and glucose. Clin Chem 64:1380–1393. https://doi.org/10.1373/clinchem.2018.288415
Carobene A, Guerra E, Locatelli M et al (2018) Providing correct estimates of biological variation’ not an easy task. The example of S100-protein and neuron-specific enolase. Clin Chem 64:1537–1539. https://doi.org/10.1373/clinchem.2018.292169
Røraas T, Støve B, Petersen PH, Sandberg S (2016) Biological variation: the effect of different distributions on estimated within-person variation and reference change values. Clin Chem 62:725–736. https://doi.org/10.1373/clinchem.2015.252296
Snedecor GW, Cochran WG (1989) Statistical methods, 8th edn. Iowa State University Press, Iowa City (IA)
Cochran WG (1941) The distribution of the largest of a set of estimated variances as a fraction of their total. Ann Hum Genet 11:47–52
Dixon WJ (1953) Processing data for outliers. Biometrics 9:74. https://doi.org/10.2307/3001634
Burdick RK, Borror CM, Montgomery DC (2005) Design and analysis of gauge R and R studies: making decisions with confidence intervals in random and mixed ANOVA models (ASA-SIAM series on statistics and applied probability)title. ASA-SIAM, Philadelphia
Diez-Perez A, Naylor KE, Abrahamsen B, Agnusdei D, Brandi ML, Cooper C, Dennison E, Eriksen EF, Gold DT, Guañabens N, Hadji P, Hiligsmann M, Horne R, Josse R, Kanis JA, Obermayer-Pietsch B, Prieto-Alhambra D, Reginster JY, Rizzoli R, Silverman S, Zillikens MC, Eastell R, Adherence Working Group of the International Osteoporosis Foundation and the European Calcified Tissue Society (2017) International Osteoporosis Foundation and European calcified tissue society working group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int 28:767–774. https://doi.org/10.1007/s00198-017-3906-6
Lorentzon M, Branco J, Brandi ML, Bruyère O, Chapurlat R, Cooper C, Cortet B, Diez-Perez A, Ferrari S, Gasparik A, Herrmann M, Jorgensen NR, Kanis J, Kaufman JM, Laslop A, Locquet M, Matijevic R, McCloskey E, Minisola S, Pikner R, Reginster JY, Rizzoli R, Szulc P, Vlaskovska M, Cavalier E (2019) Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther 36:2811–2824. https://doi.org/10.1007/s12325-019-01063-9
Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, Trenti T, Kanis JA (2011) International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine Position on bone marker standards in osteoporosis. Clin Chem Lab Med 49:1271–1274. https://doi.org/10.1515/CCLM.2011.602
Singer FR, Eyre DR (2008) Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med 75:739–750. https://doi.org/10.3949/ccjm.75.10.739
Ivaska KK, Hentunen TA, Vääräniemi J, Ylipahkala H, Pettersson K, Väänänen HK (2004) Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J Biol Chem 279:18361–18369. https://doi.org/10.1074/jbc.M314324200
Cloos PAC, Christgau S (2004) Characterization of aged osteocalcin fragments derived from bone resorption. Clin Lab 50:585–598
Biver E, Chopin F, Coiffier G et al (2012) Bone turnover markers for osteoporotic status assessment? A systematic review of their diagnosis value at baseline in osteoporosis. Jt Bone Spine 79:20–25. https://doi.org/10.1016/j.jbspin.2011.05.003
Lee AJ, Hodges S, Eastell R (2000) Measurement of osteocalcin. Ann Clin Biochem 37:432–446. https://doi.org/10.1258/0004563001899573
Garnero P, Mulleman D, Munoz F, Sornay-Rendu E, Delmas PD (2003) Long-term variability of markers of bone turnover in postmenopausal women and implications for their clinical use: the OFELY study. J Bone Miner Res 18:1789–1794. https://doi.org/10.1359/jbmr.2003.18.10.1789
Cavalier E, Lukas P, Carlisi A et al (2013) Aminoterminal propeptide of type I procollagen (PINP) in chronic kidney disease patients: the assay matters. Clin Chim Acta 425:117–118. https://doi.org/10.1016/j.cca.2013.07.016
Cavalier E, Eastell R, Jørgensen NR et al (2019) A multicenter study to evaluate harmonization of assays for N-terminal propeptide of type I procollagen (P1NP): a report from the IFCC-IOF Joint Committee for Bone Metabolism. Clin Chem Lab Med
Vasikaran SD, Bhattoa HP, Eastell R, et al. (2020) Harmonization of commercial assays for PINP; the way forward. Osteoporos. Int.
Woitge HW, Friedmann B, Suttner S, Farahmand I, Müller M, Schmidt-Gayk H, Baertsch P, Ziegler R, Seibel MJ (1998) Changes in bone turnover induced by aerobic and anaerobic exercise in young males. J Bone Miner Res 13:1797–1804. https://doi.org/10.1359/jbmr.1998.13.12.1797
Maïmoun L, Manetta J, Couret I, Dupuy AM, Mariano-Goulart D, Micallef JP, Peruchon E, Rossi M (2006) The intensity level of physical exercise and the bone metabolism response. Int J Sports Med 27:105–111. https://doi.org/10.1055/s-2005-837621
Morovat A, Catchpole A, Meurisse A et al (2013) IDS iSYS automated intact procollagen-1-Nterminus pro-peptide assay: method evaluation and reference intervals in adults and children. Clin Chem Lab Med 51:2009–2018. https://doi.org/10.1515/cclm-2012-0531
Christgau S, Bjarnason NH, Rigault M et al (1998) Intra-individual variation and response to anti-resorptive therapy assessed by bone resorption measurements with the serum CTx™ one step ELISA. Ligand Assay 3:200–205
Rogers A, Glover SJ, Eastell R (2009) A randomised, double-blinded, placebo-controlled, trial to determine the individual response in bone turnover markers to lasofoxifene therapy. Bone 45:1044–1052. https://doi.org/10.1016/j.bone.2009.07.089
Naylor KE, Jacques RM, Paggiosi M, Gossiel F, Peel NF, McCloskey E, Walsh JS, Eastell R (2016) Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 27:21–31. https://doi.org/10.1007/s00198-015-3145-7
Souberbielle J-C, Prié D, Piketty M-L, Rothenbuhler A, Delanaye P, Chanson P, Cavalier E (2017) Evaluation of a new fully automated assay for plasma intact FGF23. Calcif Tissue Int 101:510–518. https://doi.org/10.1007/s00223-017-0307-y
Smith ER, Cai MM, McMahon LP, Holt SG (2012) Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 97:3357–3365. https://doi.org/10.1210/jc.2012-1811
Dalmeijer GW, van der Schouw YT, Vermeer C et al (2013) Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem 24:624–628. https://doi.org/10.1016/j.jnutbio.2012.02.012
Cranenburg ECM, Vermeer C, Koos R, Boumans ML, Hackeng TM, Bouwman FG, Kwaijtaal M, Brandenburg VM, Ketteler M, Schurgers LJ (2008) The circulating inactive form of matrix Gla protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc Res 45:427–436. https://doi.org/10.1159/000124863
Evenepoel P, Claes K, Meijers B et al (2018) Poor vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J Bone Miner Res. https://doi.org/10.1002/jbmr.3608
Krueger T, Schlieper G, Schurgers L et al (2014) Vitamin K1 to slow vascular calcification in haemodialysis patients (VitaVasK trial): a rationale and study protocol. Nephrol Dial Transplant 29:1633–1638. https://doi.org/10.1093/ndt/gft459
van Ballegooijen A, Beulens J, Schurgers L et al (2019) Effect of 6-month vitamin D supplementation on plasma matrix Gla protein in older adults. Nutrients 11:231. https://doi.org/10.3390/nu11020231
Acknowledgments
The authors would like to thank IDS and DiaSorin for providing the reagents and all study participants and other EuBIVAS partners for their essential contribution to the project: Gerhard Barla, Bill Bartlett, Giulia Cajano, Niels Jonker, Mario Plebani, Thomas Røraas, Una Ørvim Sølvik, Marit Sverresdotter Sylte, Mustafa Serteser, Francesca Tosato, and Ibrahim Unsal; the International Osteoporosis Foundation for endorsing the paper; the Members of the IOF-IFCC Committee on Bone Metabolism who have critically read and improved the paper: Kristina Åkesson (Lund, Sweden), Harjit Pal Bhattoa (Debrecen, Hungary), Olivier Bruyère (Liège, Belgium), Cyrus Cooper (Southampton, UK), Richard Eastell (Sheffield, UK), Patrick Garnero (Lyon, France), Annemieke Heijboer (Amsterdam, The Netherlands), Niklas Rye Jorgensen (Copenhagen, Denmark), John Kanis (Sheffield, UK), Konstantinos Makris (Athens, Greece), Candice Z. Ulmer (Atlanta, USA), and Samuel Vasikaran (Murdoch, Australia); as well as the IFCC Scientific Division Executive Committee.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Etienne Cavalier is consultant for IDS and DiaSorin. Pierre Lukas, Michela Bottani, Aasne Arsand, Ferruccio Cerriotti, Abdurrahman Coskun, Jorge Diaz-Garzon, Pilar Fernandez-Calle, Elena Guerra, Massimo Locatelli, Sverre Sandberg, and Anna Carobene declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cavalier, E., Lukas, P., Bottani, M. et al. European Biological Variation Study (EuBIVAS): within- and between-subject biological variation estimates of β-isomerized C-terminal telopeptide of type I collagen (β-CTX), N-terminal propeptide of type I collagen (PINP), osteocalcin, intact fibroblast growth factor 23 and uncarboxylated-unphosphorylated matrix-Gla protein—a cooperation between the EFLM Working Group on Biological Variation and the International Osteoporosis Foundation-International Federation of Clinical Chemistry Committee on Bone Metabolism. Osteoporos Int 31, 1461–1470 (2020). https://doi.org/10.1007/s00198-020-05362-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05362-8