Abstract
Summary
The central and peripheral skeleton was characterised using imaging techniques during 104 weeks of teriparatide treatment. Teriparatide exerts differential effects on the central and the peripheral skeleton. Overall, we did not observe a change in total body bone mineral. Our conclusions are constrained by the study limitations.
Introduction
Teriparatide stimulates bone formation and resorption and therefore can cause bone gain and loss. We simultaneously characterised the central and peripheral skeleton using imaging techniques to better understand the mechanism of action of teriparatide.
Methods
Postmenopausal, osteoporotic women (n = 20, 65.4 ± 5.5 years) were recruited into a 104-week study of teriparatide. Imaging techniques included DXA, quantitative computed tomography (QCT), and high-resolution peripheral quantitative computed tomography (HR-pQCT).
Results
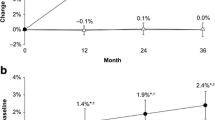
Total lumbar spine areal bone mineral content (aBMC) (+ 11.2%), total lumbar spine areal bone mineral density (aBMD) (+ 8.1%), subregional thoracic spine aBMD (+ 7.5%), lumbar spine aBMC (+ 23.5%), lumbar spine aBMD (+ 11.9%), pelvis aBMC (+ 9.3%), and pelvis aBMD (+ 4.3%) increased. However, skull aBMC (− 5.0%), arms aBMC (− 5.1%), legs aBMC (− 2.9%), and legs aBMD (− 2.5%) decreased. Overall, we did not observe a change in total body bone mineral.
Increases in L1–L3 volumetric BMD (vBMD) (+ 28.5%) occurred but there was no change in total proximal femur vBMD.
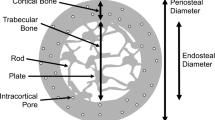
Radius and tibia cortical vBMD (− 3.3 and − 3.4%) and tissue mineral density (− 3.2 and − 3.8%) decreased and there was an increase in porosity (+ 21.2 and + 10.3%). Tibia, but not radius, trabecular inhomogeneity (+ 3.2%), and failure load (+ 0.2%) increased, but cortical thickness (− 3.1%), area (− 2.9%), and pore volume (− 1.6%) decreased.
Conclusions
Teriparatide exerts differential effects on the central and the peripheral skeleton. Central trabecular vBMD (L1–L3) is improved, but there is a concomitant decrease in peripheral cortical vBMD and an increase in porosity. Overall, we did not observe a change in total body bone mineral. We acknowledge that our conclusions may be speculative and are constrained by the technical limitations of the imaging techniques used, the lack of a control group, and the small sample size studied.

Similar content being viewed by others
References
Chen P, Satterwhite JH, Licata AA et al (2006) Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res 20:962–970
Ma YL, Zeng Q, Donley DW, Ste-Marie LG, Gallagher JC, Dalsky GP, Marcus R, Eriksen EF (2006) Teriparatide increase bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res 21:855–864
Eastell R, Krege JH, Chen P, Glass EV, Reginster JY (2006) Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr Med Res Opin 22:61–66
Genant HK, Engelke K, Bolognese MA, Mautalen C, Brown JP, Recknor C, Goemaere S, Fuerst T, Yang YC, Grauer A, Libanati C (2017) Effects of romosozumab compared with teriparatide on bone mineral density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res 32:181–187
Kleerekoper M, Greenspan SL, Lewiecki M et al (2014) Assessing the effects of teriparatide treatment on bone mineral density, bone microarchitecture, and bone strength. J Bone Joint Surg Am 96(11):e90
Miyauchi A, Matsumoto T, Sugimoto T, Tsujimoto M, Warner MR, Nakamura T (2010) Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone 47:493–502
Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, Audran M, Barker C, Anastasilakis AD, Fraser WD, Nickelsen T, EUROFORS Investigators (2008) Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 23:1591–1600
McClung MR, San MJ, Miller PD et al (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768
Slovik DM, Neer RM, Potts JT Jr (1981) Short-term effects of synthetic human parathyroid hormone-(1–34) administration on bone mineral metabolism in osteoporotic patients. J Clin Invest 68(5):1261–1271
Gonnelli S, Martini G, Caffarelli C et al (2006) Teriparatide’s effects on quantitative ultrasound parameters and bone density in women with established osteoporosis. Osteoporos Int 17(10):1524–1531
Graeff C, Timm W, Nickelsen TN, Farrerons J, Marín F, Barker C, Glüer CC, EUROFORS High Resolution Computed Tomography Substudy Group (2007) Monitoring teriparatide-associated changes in vertebral microstructure by high-resolution CT in vivo: results from the EUROFORS study. J Bone Miner Res 22:1426–1433
Borgreffe J, Graeff C, Nickelson TN, Marin F, Glüer CC (2010) Quantitative computed tomographic assessment of the effects of 24 months of teriparatide treatment on 3D femoral neck bone distribution, geometry, and bone strength: results from the EUROFORS study. J Bone Miner Res 25:472–481
Keaveny TM, Crittenden DB, Bolognese MA, Genant HK, Engelke K, Oliveri B, Brown JP, Langdahl BL, Yan C, Grayer A, Libanati C (2017) Greater gains in spine and hip strength for romosozumab compared with teriparatide in postmenopausal women with low bone mass. J Bone Mineral Res 32:1956–1962
Graeff C, Chevalier Y, Charlebois M, Varga P, Pahr D, Nickelsen TN, Morlock MM, Glüer CC, Zysset PK (2009) Improvements in vertebral body strength under teriparatide treatment assess in vivo by finite element analysis: results from the Eurofors study. J Bone Miner Res 24(10):1672–1680
McDonald HM, Nishiyama KK, Hanley DA, Boyd SK (2010) Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int 22:357–362
Tsai JN, Uihlein AV, Burnett-Bowie S-AM, Neer RM, Zhu Y, Derrico N, Lee H, Bouxsein ML, Leder BZ (2015) Comparative effects of teriparatide, Denosumab, and combination therapy on peripheral compartmental bone density, microarchitecture and estimated strength: the DATA-HRpQCT study. J Bone Miner Res 30(1):39–45
Hansen S, Hauge EM, Jensen JEB, Brixen K (2013) Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res 28(4):736–745
Jiang G, Eastell R, Barrington NA, Ferrar L (2004) Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int 15(11):887–896
Hildebrandt EM, Manske SL, Hanley DA, Boyd SK (2016) Bilateral asymmetry of radius and tibia bone macroarchitecture and microarchitecture: a high-resolution peripheral quantitative computed tomography study. J Clin Densitom 19(2):250–254
Sode M, Burghardt AJ, Pialat J-B, Link TM, Majumdar S (2011) Quantitative characterisation of subject motion in HR-pQCT images of the distal radius and tibia. Bone 48(6):1291–1297
Engelke K, Stampa B, Timm W (2012) Short-term in vivo precision of BMD and parameters of trabecular architecture at the distal forearm and tibia. Osteoporos Int 23(8):2151–2158
Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK (2010) Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone 47(3):519–528
Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD (2008) Finite element analysis based on in vivo HR-pQCT images of the radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23(3):392–399
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312(7041):1254–1259
Moore AE, Blake GM, Taylor KA, Rana AE, Wong M, Chen P, Fogelman I (2010) Assessment of regional changes in skeletal metabolism following 3 and 18 months of teriparatide treatment. J Bone Miner Res 25(5):960–967
Finkelstein JS, Wyland JJ, Lee H, Neer RM (2010) Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 95(4):1838–1845
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344(19):1434–1441
Orwell ES, Scheele WH, Paul S et al (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density on men with osteoporosis. J Bone Miner Res 18:9–17
Yu EW, Neer RM, Lee H, Wyland JJ, de la Paz AV, Davis MC, Okazaki M, Finkelstein JS (2011) Time-dependent changes in skeletal response to teriparatide: escalating versus constant dose teriparatide (PTH 1–34) in osteoporotic women. Bone 48(4):713–719
Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, Jasqui S, Donley DW, Dalsky GP, Martin JS, Eriksen EF (2005) Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res 20:1244–1253
Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC (1999) Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 104:439–446
Boonen S, Marin F, Obermayer-Pietsch et al for the EUROFORS investigators (2008) Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 93(3):852–860
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751
Whitmarsh T, Treece GM, Gee AH, Poole KES (2015) Mapping bone changes at the proximal femoral cortex of postmenopausal women in response to alendronate and teriparatide alone, combined or sequentially. J Bone Miner Res 30(7):1309–1318
Uusi-Rasi K, Semanick LM, Zanchetta JR et al (2005) Effects of teriparatide [rhPTH (1-34)] treatment on structural geometry of the proximal femur in elderly osteoporotic women. Bone 36:948–958
Lindsay R, Krege JH, Marin F, Jin L, Stepan JJ (2016) Teriparatide for osteoporosis: importance of the full course. Osteoporos Int 27(8):2395–2410
Funding
This work was funded by the National Institute for Health Research (NIHR) via its Biomedical Research Units Funding Scheme and the NIHR Sheffield Clinical Research Facility. The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health (DoH). Teriparatide was provided by Eli Lilly and Company, Basingstoke, UK to Professor R Eastell (PI) through an Investigator-Initiated Trial (IIT). We acknowledge the Clinical Trials Research Unit, School of Health and Related Research (ScHARR), the University of Sheffield for study data management and statistical advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
MAP, LY, and DB state no disclosure. JSW has received speaker’s honoraria from Lilly, grant funding from Alexion and Immunodiagnostic Systems, donation of drug from Prostrakan for a clinical trial, consulting fees from Shire and Mereo Biopharma. NP has received speaker’s honoraria and participated in advisory board activities for Eli Lilly and Prostraken. EVM has received consultancy payments from Merck, UCB, Consilient, speaker fees from Bayer, Consilient, GSK, Amgen, UCB, Roche, Servier, Lilly, and payment for development from Lilly. RE has received consultancy payments, speaker fees, and grant funding from Eli Lilly and Company. Teriparatide was provided by Eli Lilly and Company, Basingstoke, UK to the University of Sheffield through an Investigator-Initiated Trial (IIT) award. The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health (DoH).
Rights and permissions
About this article
Cite this article
Paggiosi, M.A., Yang, L., Blackwell, D. et al. Teriparatide treatment exerts differential effects on the central and peripheral skeleton: results from the MOAT study. Osteoporos Int 29, 1367–1378 (2018). https://doi.org/10.1007/s00198-018-4445-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4445-5