Abstract
Purpose
The use of heat and moisture exchangers (HME) during noninvasive ventilation (NIV) can increase the work of breathing, decrease alveolar ventilation, and deliver less humidity in comparison with heated humidifiers (HH). We tested the hypothesis that the use of HH during NIV with ICU ventilators for patients with acute respiratory failure would decrease the rate of intubation (primary endpoint) as compared with HME.
Methods
We conducted a multicenter randomized controlled study in 15 centers. After stratification by center and type of respiratory failure (hypoxemic or hypercapnic), eligible patients were randomized to receive NIV with HH or HME.
Results
Of the 247 patients included, 128 patients were allocated to the HME group and 119 to the HH group. Patients were comparable at baseline. The intubation rate was not significantly different: 29.7 % in the HME group and 36.9 % in the HH group (p = 0.28). PaCO2 did not significantly differ between the two arms, even in the subgroup of hypercapnic patients. No significant difference was observed for NIV duration, ICU and hospital LOS, or ICU mortality (HME 14.1 vs. HH 21.5 %, p = 0.18).
Conclusions
In this study, the short-term physiological benefits of HH in comparison with HME during NIV with ICU ventilators were not observed, and no difference in intubation rate was found. The physiologic effects may have been obscured by leaks or other important factors in the clinical settings. This study does not support the recent recommendation favoring the use of HH during NIV with ICU ventilators.
Similar content being viewed by others
Introduction
Recent recommendations have been published favoring the use of heated humidifiers (HH) during noninvasive ventilation (NIV) [1]. There are few physiologic studies that support these recommendations but no study reporting the impact of the humidification device use during NIV for acute respiratory failure on patient outcome [2]. Although the upper airways are not bypassed, several arguments plead for the humidification of delivered gases during NIV. The inspiratory gases are dry with ICU ventilators, respiratory rates are high, and mouth breathing is frequent during NIV [3, 4]. Mucosal dryness is also a frequent complaint during NIV [5, 6] and a good tolerance of the technique is necessary for its success [7, 8]. Most of all, in patients receiving NIV, bronchial hyperreactivity may be increased by dry medical gases [9–11].
It was shown in short-term studies that HH can deliver gases with higher water content than heat and moisture exchangers (HME) during NIV, especially in the case of leaks [12]. It was also shown with short-term physiologic studies that HH improved alveolar ventilation and CO2 elimination [13, 14] and reduced the work of breathing [15] when compared with HME. These results are related to the additional instrumental dead space on the circuit with HME that must be placed after the Y-piece on the patient’s side [15]. Interestingly, the lack of CO2 removal during NIV in COPD patients was found to be a major criterion for NIV failure [16–20].
Therefore, the combined physiologic data plead for the use of HH during NIV. However, taking into account the economic implications and ergonomic aspects, the widespread use of HH can not be promoted without obtaining clinical studies demonstrating an impact on important outcome data such as the rate of intubation, the ICU length of stay, or the mortality. The purpose of this randomized controlled multicenter study was to test the hypothesis that NIV delivered via ICU ventilators with HH is associated with a reduced rate of intubation in comparison to HME [21].
Methods
The study was conducted in 15 medical, surgical, or mixed ICUs, in university hospitals in France, Tunisia, Italy, and Canada. The participating centers were experienced in the use of NIV, with implementation of this technique on average 8 ± 3 years before the start of the study. The study centers received approval from an ethics committee. Written informed consent was obtained from all participants or their next of kin.
Patients
Inclusion criteria were age between 18 and 85 years, exacerbation of dyspnea for less than 2 weeks, and presence of at least two of the following criteria: respiratory rate above 25 breaths/min, SaO2 below 90 % (ambient air or oxygen), arterial pH less than 7.35. The exclusion criteria were need for immediate intubation, major facial deformity, high probability of a surgical procedure, prior episode of invasive mechanical ventilation of more than 48 h during the same hospital stay, pneumothorax, refusal of intubation by the patient, decision not to resuscitate or not to intubate the patient already known at inclusion, pregnancy, organ failure other than lungs based on absence of organ dysfunctions and/or infection (ODIN) score [22]. Registration of inclusion and exclusion was carried out using data from each site concerning patients requiring NIV during the year of the study.
Study protocol
Patients were prospectively randomized into the HH arm (MR850, Fisher & Paykel) or HME arm (Hygrobac, Tyco Healthcare; dead space 95 ml [23]). Central randomization was conducted by phone with a voice recognition system and stratification was performed on the basis of the study center and on the type of respiratory failure as follows: “respiratory acidosis”, pH less than 7.35 and PaCO2 greater than 45 mmHg at baseline; or “hypoxemia”, SaO2 no greater than 90 % in ambient air or oxygen. If respiratory acidosis was present with hypoxemia, respiratory acidosis was prioritized.
The humidification device allocated at randomization was used during all NIV sessions, until intubation or NIV cessation. All other technical aspects of NIV (including same oronasal mask and the absence of flex tube use) were standardized for each group. Only ICU ventilators were used to deliver NIV. The recommended ventilatory mode was pressure support with the aim of obtaining an expired tidal volume of at least 7 ml/kg. It was also recommended to perform more than 3 h of NIV within the first 6 h following inclusion and more than 6 h during the first 24 h.
Endpoints
The primary endpoint of the study was the intubation rate. Criteria for intubation were standardized and based on previous publications [24] (criteria detailed in the electronic supplementary material).
The secondary endpoints were intubation rate in the hypoxemia and respiratory acidosis groups, physiological parameters (respiratory rate, hemodynamic parameters, arterial blood gas), NIV tolerance (mucosal dryness), the total length of mechanical ventilation, the length of intensive care unit and hospital stay, and intensive care unit and hospital mortality rates.
Data collection
The demographic characteristics (age, sex, BMI, comorbidities), the indication for NIV, the severity scores (SAPS II [25], LODS [26], McCabe [27]), and the physiologic characteristics were recorded at baseline.
Daily collected data were standard respiratory and hemodynamic physiologic data, as well as an assessment of upper respiratory tract mucosal dryness and duration of NIV.
Statistical analysis
The expected rate of intubation for patients undergoing NIV was around 40 % [7, 8]. A sample of 125 patients in each group was thus selected to detect an absolute reduction of 15 % in intubation rate, with a β risk of 0.2 and an α risk of 0.05, with a bilateral test. The analysis was performed on an intention to treat basis. Results are given as median (25th–75th interquartiles). Proportions were compared using the Chi square test or Fisher’s exact test. To compare the continuous variables, the Mann–Whitney U test was used for non-normally distributed variables or a t test was used when appropriate. Survival curves were estimated by the Kaplan–Meier method and compared using log-rank tests. Administrative censoring was performed at day 28. We performed an adjusted analysis with the Cox regression model with treatment arm and stratification group (hypoxemia and hypercapnia) as categorical covariables with interactions evaluation. p values of 0.05 or less were considered significant.
Results
Demographic characteristics and baseline physiologic data
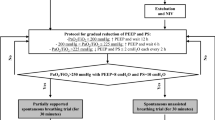
Patients were included between November 2002 and December 2003. During the 13 months of the study, 249 patients were randomized. Two patients were excluded from the analysis, one for inclusion error and one because inclusion criteria were not met. Thus, data from 247 patients were analyzed, 119 in the HH arm and 128 in the HME arm (Fig. 1). Centers included an average of 16.6 ± 13.8 patients during this period, which represents an average of 1.4 inclusions per month per center.
The characteristics of the included patients are depicted in Table 1. Many of the baseline characteristics of the two study arms such as age, severity scores, comorbidities, and indications for NIV were similar. In the hypoxemia subgroup, LODS was significantly greater in the HH arm (Table 1).
Main arterial blood gases values and physiological characteristics were similar in the two arms at baseline (Table 2). In the hypoxemia subgroup, median PaCO2 was higher in the HME arm and the bicarbonate levels were lower in the HH arm (Table 2).
Primary endpoint
There was no significant difference in the intubation rate between HH and HME for the entire population (36.9 vs. 29.7 % respectively, p = 0.28, Table 3). Criteria for intubation met in both groups are reported in Table 4.
Secondary endpoints
In the respiratory acidosis group, the intubation rate was very similar between HH and HME, whereas there was a nonstatistically significant difference in the rate of intubation with HH in the hypoxemia group (41 vs. 26 %, p = 0.14, Table 3).
The NIV duration was the same in HH and HME groups. No significant difference was found regarding either ICU or hospital length of stay or mortality (Table 3, Fig. 2). Mortality was increased at day 28 with HH in the hypoxemia subgroup but the difference did not reach statistical significance. Five patients in the HH group vs. one in the HME group died before reintubation (p = 0.11).
There was no difference in PaCO2 between the two arms, even in the respiratory acidosis group. In this group, the PaCO2 was lower in the HH arm at 3 h but the difference did not reach statistical significance (66 vs. 72 mmHg, p = 0.08). In the same arm, the diastolic blood pressure was significantly lower in the HH arm at 3 h of NIV. The main ventilator settings (pressure support level, PEEP, and FiO2) did not differ (see electronic supplementary material).
There was no significant difference with regard to the mucosal dryness in both groups from day 1 through day 7 with median values between 0 and 1. The percentage of scores of 2 or 3 (witnessed significant mucosal dryness) was similar and moderate in both groups from day 1 through day 7. From day 1 to day 7, the mean number of patients with significant mucosal dryness was 13.8 % in the HH group and 13.2 % in the HME group.
Discussion
This is the first multicenter randomized study that assessed the impact of the type of humidification system used on the success rate of NIV delivered for acute respiratory failure with ICU ventilators. Surprisingly, the expected physiological effects of the two systems were not present and, overall, the intubation rate was not influenced by the humidification system used. The hypothesis of the superiority of heated humidifiers relying on previous physiological studies was not confirmed. The results of the present study do not support recent recommendations favoring the use of HH during NIV [1].
Our initial hypothesis was a reduction in the rate of intubation with HH in comparison with HME, in line with the data obtained during several previously conducted physiologic studies [12–15]. Indeed, the lower dead space involved in HH is associated with a reduction in respiratory effort [15], minute ventilation, and an increase in alveolar ventilation (decrease in PaCO2) compared to HME [13, 14]. Our hypothesis was also supported by data showing that the lack of CO2 removal is a predictor of NIV failure in COPD patients [16–20]. In addition, these studies demonstrated a higher level of gas humidification with HH [12]. Yet, no impact on the rate of intubation was noted in our study even in the group of hypercapnic patients.
There is currently no clinical study assessing the impact of humidification device used during NIV for acute respiratory failure that can be compared with the present study. One 12-month cross-over pilot study compared HH to HME during chronic NIV in 16 COPD patients. The authors did not find any differences for NIV compliance, tolerance, rate of hospitalization, or for most of the complications related to dry gases [2]. The authors noted more dry throat (50 vs. 36 %) and slightly but significantly increased levels of PaCO2 (52.3 ± 5.4 vs. 51.1 ± 4.2 mmHg) with HME in comparison with HH [2].
Several issues might explain the lack of impact of humidification devices on NIV success rate in this study. It may be more difficult to demonstrate under the conditions of a multicentric study some findings demonstrated within well-controlled physiological studies such as increased work of breathing, reduced CO2 elimination with HME, and higher humidification performances with HH. In this regard, we should consider the impact of the application of PEEP and of leaks, which may differ between well-controlled physiological studies and real-life administration. During real-life NIV administration, a PEEP level is frequently needed, thereby reducing the impact of the difference in dead space between humidification systems as previously shown in physiological studies. It was shown that increased work of breathing related to the additional dead space generated by HME was less marked with the application of a PEEP level of 5 cmH2O [15], possibly explained by a dead space washout due to leaks. In the present study, the median level of PEEP during the first NIV session was 5 cmH2O in both groups; thus the difference in respiratory work of breathing may have been small between the two arms. We analyzed the rate of “NIV failure” (counted as death before reintubation or intubation), given that five patients in the HH group vs. one in the HME group died before intubation. The conclusions are not modified when death without intubation is considered as a NIV failure. We also performed an adjusted analysis with the Cox regression model with the treatment arm and stratification group as categorical covariables which did not modify the conclusions. We observed no significant impact of the humidification device on the arterial gas changes, contrasting with previous physiological studies [14, 15] and with a clinical study [2]. In the respiratory acidosis arm, there was only a nonsignificant difference towards greater PaCO2 clearance at the third hour following inclusion with HH as compared to HME (66 vs. 72 mmHg, p = 0.08) (see electronic supplementary material). It was previously shown that the correction of respiratory acidosis was a major determinant of the NIV success in COPD patients [16–20]. However, we did not observe a faster CO2 elimination as could be obtained by reducing the dead space with HH [13, 14]. Again, the most likely explanation is the presence of end expiratory leaks generating a washout effect of any CO2 in the mask. In addition, we observed that a decrease in diastolic arterial pressure and hemodynamic effects could in part explain the poor results obtained with HH. It is difficult, however, to incriminate a decrease of PaCO2 which has been shown to be associated with a decrease in cardiac output during NIV in COPD patients [28].
In the subgroup of patients with hypoxemia, a nonstatistically significant difference for increased intubation and mortality rates with HH was found. The subgroup of hypoxemic patients with HH had the highest LODS scores, a lower PaCO2 suggesting a higher minute ventilation, a lower bicarbonate level, and a higher need for FiO2—all these differences being significant. The higher apparent severity of this subgroup may therefore explain the results. These results rely on analyses conducted on small numbers of patient. Consequently the differences may be due to chance and the sample size for these subgroup analyses does not allow a definitive conclusion. However, these findings raise some questions and at least do not support the routine use of heated humidifiers in all patients during NIV as recently recommended [8].
Another interesting result of this study was that no significant difference was observed between the two groups regarding mucosal dryness, despite presumed higher humidification performances of HH. Indeed, severe dryness frequency was low, ranging from 8.5 to 17.5 % between day 1 and day 7. The better humidification performances of the HH have been shown in favorable conditions [29]. These performances may have been reduced as a result of suboptimal conditions which exist in a real-life setting, especially the high ambient temperatures related to the lack of air conditioning in several participating centers [29]. In a previous study conducted in healthy subjects moderate levels of humidity obtained with HME (even in the case of leaks) were as comfortable as higher humidity obtained with HH [12]. These data show that both humidification devices seem capable under the study conditions of reducing this frequently reported complication [5, 6].
The strength of the current study was the control for usual confounding factors that could bias the study favoring therefore one arm over the other. The median level of pressure support was not significantly different between both arms: 12 (11–16) cmH2O in HME arm vs. 14 (12–15) cmH2O in HH arm (p = 0.24). Moreover, NIV daily duration was equivalent in both groups, except for the second study day during which patients received a few minutes extra NIV within the HH arm (8.2 vs. 8.0 h, p = 0.02). Such a difference is probably not clinically relevant. It is also important to note that participating centers were homogeneous and had several years of experience of NIV. However, it was not possible to control other factors that may have a prominent role on NIV outcome such as the level of leaks, comfort, or patient–ventilator asynchronies, which may be more important than humidification in explaining NIV success or failure. Of note, none of the ventilators used had an “NIV” mode.
There are several limitations to this study. We acknowledge that the study was underpowered and definitive conclusions are not possible; this is especially true for subgroups analysis. Only two types of humidification systems were used. Other systems, especially HME with low dead space, may influence the impact on PaCO2 clearance [30]. Similarly, we did not include a third arm without a humidification system [31]. This option might have been feasible for short NIV periods using turbine ventilators and at low levels of FiO2; however, turbine ventilators are infrequently used in Europe [8], as confirmed by the present study (see electronic supplementary material). Thus, the use of a humidification system to avoid dry gas delivery is well justified, especially in NIV patients that frequently feature bronchial hyperreactivity [9, 10] and which can be further increased by the use of dry gases [11]. In the present study, we used an HME with a relatively large dead space, and these results may not be extrapolated to HMEs with a lower dead space. We did not record ventilator settings such as inspiratory or expiratory triggers and pressurization ramp which may potentially have a clinical impact. We thus do not know if these settings differed between groups. Finally, another potential limitation is that this was an open label study. Indeed, it is impossible to envisage driving this type of study blindly. To reduce the possible bias related to this methodology, we used well-defined criteria for our primary endpoint of intubation.
In conclusion, the humidification device used during NIV with ICU ventilators did not have a statistically significant impact on the success rate of this technique in this study. In addition, no difference in the patients’ mucosal dryness was reported with HH in comparison with HME. Our results suggest that despite a strong physiologic rationale supporting the use of heated humidifiers during NIV, this device cannot be recommended as a first-line treatment in all patients with acute respiratory failure. The use of an HME (ideally with a low dead space) while removing the additional dead space (flex tubing) seems to be acceptable in light of the results of this study. In the presence of persistent high PaCO2 levels associated with threatening encephalopathy, the reduction of dead space with an HH may be considered.
References
American Association for Respiratory Care, Restrepo RD, Walsh BK (2012) Humidification during invasive and noninvasive mechanical ventilation. Respir Care 57:782–788
Nava S, Navalesi P, Gregoretti C (2009) Interfaces and humidification for noninvasive mechanical ventilation. Respir Care 54:71–84
Primiano FP Jr, Montague FW Jr, Saidel GM (1984) Measurement system for respiratory water vapor and temperature dynamics. J Appl Physiol 56:1679–1685
Sara C, Currie T (1965) Humidification by nebulization. Med J Aust 191:174–179
Hill NS (1997) Complications of noninvasive positive pressure ventilation. Respir Care 42:432–442
Mehta S, Hill NS (2001) Noninvasive ventilation. Am J Respir Crit Care Med 163:540–577
Carlucci A, Richard JC, Wysocki M, Lepage E, Brochard L (2001) Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med 163:874–880
Demoule A, Girou E, Richard JC, Taille S, Brochard L (2006) Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med 32:1747–1755
Cabanes LR, Weber SN, Matran R, Regnard J, Richard MO, Degeorges ME, Lockhart A (1989) Bronchial hyper-responsiveness to methacholine in patients with impaired left ventricular function. N Engl J Med 320:1317–1322
Hospers JJ, Postma DS, Rijcken B, Weiss ST, Schouten JP (2000) Histamine airway hyper-responsiveness and mortality from chronic obstructive pulmonary disease: a cohort study. Lancet 356:1313–1317
Fontanari P, Burnet H, Zattara-Hartmann MC, Jammes Y (1996) Changes in airway resistance induced by nasal inhalation of cold dry, dry, or moist air in normal individuals. J Appl Physiol 81:1739–1743
Lellouche F, Maggiore SM, Lyazidi A, Deye N, Taille S, Brochard L (2009) Water content of delivered gases during non-invasive ventilation in healthy subjects. Intensive Care Med 35:987–995
Lellouche F, Pignataro C, Maggiore SM, Girou E, Deye N, Taille S, Fischler M, Brochard L (2012) Short-term effects of humidification devices on respiratory pattern and arterial blood gases during noninvasive ventilation. Respir Care 57:1879–1886
Jaber S, Chanques G, Matecki S, Ramonatxo M, Souche B, Perrigault PF, Eledjam JJ (2002) Comparison of the effects of heat and moisture exchangers and heated humidifiers on ventilation and gas exchange during non-invasive ventilation. Intensive Care Med 28:1590–1594
Lellouche F, Maggiore SM, Deye N, Taille S, Pigeot J, Harf A, Brochard L (2002) Effect of the humidification device on the work of breathing during noninvasive ventilation. Intensive Care Med 28:1582–1589
Ambrosino N, Foglio K, Rubini F, Clini E, Nava S, Vitacca M (1995) Non-invasive mechanical ventilation in acute respiratory failure due to chronic obstructive pulmonary disease: correlates for success. Thorax 50:755–757
Anton A, Guell R, Gomez J, Serrano J, Castellano A, Carrasco JL, Sanchis J (2000) Predicting the result of noninvasive ventilation in severe acute exacerbations of patients with chronic airflow limitation. Chest 117:828–833
Poponick JM, Renston JP, Bennett RP, Emerman CL (1999) Use of a ventilatory support system (BiPAP) for acute respiratory failure in the emergency department. Chest 116:166–171
Putinati S, Ballerin L, Piattella M, Panella GL, Potena A (2000) Is it possible to predict the success of non-invasive positive pressure ventilation in acute respiratory failure due to COPD? Respir Med 94:997–1001
Soo Hoo GW, Santiago S, Williams AJ (1994) Nasal mechanical ventilation for hypercapnic respiratory failure in chronic obstructive pulmonary disease: determinants of success and failure. Crit Care Med 22:1253–1261
Lellouche F, L’Her E, Abrouk F, Deye N, Rabbat A, Jaber S, Fartoukh M, Conti G, Cracco C, Ricard JD, Mal H, Mentec H, Loisel F, Lacherade JC, Rodriguez P, Brochard L (2006) Impact of the humidification device on intubation rate during NIV: results of a multicenter RCT. Am J Respir Crit Care Med 3:A471
Le Gal YM, Fabre J, Barthelemy R, Vanleeuw P, Lagente M, Puel P (1994) Evaluation of a cardiopulmonary preservation method for heart–lung transplantation. Acta Biomed Ateneo Parmense 65:213–224
Lellouche F, Taille S, Lefrancois F, Deye N, Maggiore SM, Jouvet P, Ricard JD, Fumagalli B, Brochard L (2009) Humidification performance of 48 passive airway humidifiers: comparison with manufacturer data. Chest 135:276–286
Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F et al (1995) Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 333:817–822
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Le Gall JR, Klar J, Lemeshow S, Saulnier F, Alberti C, Artigas A, Teres D (1996) The logistic organ dysfunction system. A new way to assess organ dysfunction in the intensive care unit ICU scoring group. JAMA 276:802–810
McCabe WR, Jackson GG (1962) Gram-negative bacteriemia: I. Etiology and ecology. Arch Intern Med 110:847–855
Diaz O, Iglesia R, Ferrer M, Zavala E, Santos C, Wagner PD, Roca J, Rodriguez-Roisin R (1997) Effects of noninvasive ventilation on pulmonary gas exchange and hemodynamics during acute hypercapnic exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 156:1840–1845
Lellouche L, Taillé S, Maggiore SM, Qader S, L’Her E, Deye N, Brochard L (2004) Influence of ambient air and ventilator output temperature on performances of heated-wire humidifiers. Am J Respir Crit Care Med 170:1073–1079
Boyer A, Vargas F, Hilbert G, Gruson D, Mousset-Hovaere M, Castaing Y, Dreyfuss D, Ricard JD (2010) Small dead space heat and moisture exchangers do not impede gas exchange during noninvasive ventilation: a comparison with a heated humidifier. Intensive Care Med 36:1348–1354
Branson RD, Gentile MA (2010) Is humidification always necessary during noninvasive ventilation in the hospital? Respir Care 55:209–216
Acknowledgments
The humidification devices and the masks for this study were supplied free of charge by their manufacturers (Fisher & Paykel and Tyco Healthcare). The study was funded by PHRC (Programme Hospitalier de Recherche Clinique-Assistance Publique des Hôpitaux de Paris).
Conflicts of interest
F. L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; L. B., as director of the research laboratory of the Créteil Department of Medical Intensive Care Medicine, received a grant from Hudson Company of €10,000 in 2002 and, as director, a grant from Fisher & Paykel Company of €8,700 in 2001 and €10,000 in 2002 to the same laboratory. The other authors have no conflicts of interest to declare.
Ethical standards statement
The French centers and each center abroad received approval from an ethics committee. Written informed consent was obtained from all participants or their next of kin. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: The short-term physiological benefits of heated humidifiers (HH) in comparison with heat and moisture exchangers during NIV with ICU ventilators were not observed in this randomized controlled study including 247 patients, and no difference in intubation rate was found. The physiologic effects may have been obscured by leaks or other important factors in the clinical settings. This study does not support the recent recommendation favoring the use of HH during NIV.
ClinicalTrials.gov Identifier: NCT00190346.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lellouche, F., L’Her, E., Abroug, F. et al. Impact of the humidification device on intubation rate during noninvasive ventilation with ICU ventilators: results of a multicenter randomized controlled trial. Intensive Care Med 40, 211–219 (2014). https://doi.org/10.1007/s00134-013-3145-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-3145-z