Abstract
Elemental tracer concentrations of copper, lead, nickel and zinc, were assessed in the muscles of Oreochromis amphimelas and Clarias gariepinus from Lake Manyara, Tanzania, to evaluate their safety to consumers, specifically humans. Results revealed that no elemental concentrations exceeded the FAO permissible levels, indicating fish from all sites are safe for human consumption. However, based on the highest found concentration of Pb, we recommend a maximum consumption of 2.2 kg of fish from Lake Manyara per week. No significant differences were observed in the metal concentrations between the two fish species, suggesting there is no bioaccumulation in the food chain. Moreover, no significant differences were found between fish landing sites, indicating there are no regions in the lake with higher pollution. These findings indicate that PTM concentrations have not increased to toxic levels due to increased mobilisation from the catchment. Continued monitoring of potential toxic metal concentrations in fish is recommended due to endorheic nature of Lake Manyara and increasing anthropogenic activities in its catchment area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
According to URT 2010 and FAO 2020, the world’s fisheries play a significant economic role in the production of food, jobs creation, income, and tax revenue. Globally, around 60 million people are employed in fishing, with the majority in developing nations (FAO 2018). In addition to these economic advantages, fish is important as a source of protein, essential minor nutrients, and nutritive non-saturated fatty acids and Omega-3 that aid in lowering blood cholesterol and preventing heart problems (Erkkilä et al. 2004). However, spills and discharges from industrial, agricultural, and urban sources that contaminated soils, groundwater, and surface water in recent decades have had an adverse effect on most aquatic ecosystems. Heavy metals like cadmium, mercury, lead, zinc and copper are of particular concern because of their toxicity, persistence in the environment, and tendency to accumulate in food chains (Tam and Wong 2000). Fish are considered to be good indicators of water pollution by metals and the degree of biological effects to humans, aquatic species, and the associated food web (Klaverkamp et al. 1984). Metal pollution can impact societies and ecosystems through direct toxicity to humans and aquatic life, as well as indirect toxicity through accumulations of metals in the aquatic food web. The Lake Manyara basin, which includes Lake Manyara National Park, is of great ecological and socio-economic value. The diversity of ecosystems and high biodiversity support agriculture, fisheries, pastoralism, and ecotourism (Janssens de Bisthoven et al. 2020). However, the lake itself is threatened by rising anthropogenic pressures in its watershed. Due to the fact that Lake Manyara is an endorheic system without an outflow, pollution loading poses a particular risk. Agricultural areas have increased from about 10 to 25% over the last three decades, which has increased the basin’s potential for erosion (Wynants et al. 2018). Furthermore, agro-pastoral systems are degrading as a result of unsustainable agricultural methods and overgrazing (Wynants et al. 2021b). The rapid downstream movement of eroded sediments through intricate networks of ephemeral gullies has caused Lake Manyara’s sedimentation rates to quadruple (Wynants et al. 2020). In coffee plantation soils, copper (Cu) concentrations have been found to be relatively higher (Wynants et al. 2021a), which is most likely a result of the use of Cu-based fungicides. Additionally, the Minjingu mine, one of Eastern Africa’s most significant phosphate mines, is situated about 5 km southeast of the lake, raising the possibility of phosphate runoff and other harmful byproducts (Banzi et al. 2000).
Although the effects of eutrophication and toxic algal blooms on the ecosystem of Lake Manyara have already been thoroughly studied (Nonga et al. 2011), it is unknown how potentially toxic metals (PTMs) accumulate in fish. As been shown by Mng’ong’o et al., (2021), PTM concentrations in other Tanzanian water bodies have increased in recent decades as a result of their connection to eroded sediments, direct runoff of agricultural additives, or discharge from mines and sewage treatment plants. Since the presence of commercial and subsistence fisheries in Lake Manyara, it is therefore crucial to look into the levels of PTMs in fish from Lake Manyara in order to protect the health of consumers due to the negative health effects that eating polluted fish may have. The endemic Manyara tilapia (Oreochromis amphimelas) from Lake Manyara and the African catfish (Clarias gariepinus) were the subjects of this study, in which we examined the levels of PTMs in edible parts of both species. Manyara tilapia are microphagous (Shechonge et al. 2019), while African catfish are omnivores that become more predatory when they mature (Lemmens et al. 2017). In this study, one non-essential element (lead) and three micronutrients (copper, nickel, and zinc) were examined for screening purposes. In the crust of the earth, copper (Cu) naturally occurs as oxides and sulfides, and occasionally as metallic copper. Cu is related to other metals like cadmium, silver, tin, and zinc. It is a micronutrient that many different enzymes and other cell components need in very small concentrations to support essential processes in all living things. Excessive intakes however, can result in gastrointestinal symptoms and liver damage. (Demirezen and Uruc 2006; Ivo et al. 2013; Lee et al. 2021). Natural occurrences of lead (Pb) in the crust of the earth typically occur as a significant component of minerals. However, refinery of Pb and its global use has resulted in significant environmental contamination and health issues due to its high toxicity when concentrated (D’Souza et al. 2011, Mărginean et al. 2016). Pb negatively affects majority of the body’s major organ systems, especially the hematopoietic, renal, nervous, and cardiovascular systems, and even small concentrations of Pb have been shown to cause irreversible health effects. Pb also has no known biological function in the body (Flora et al. 2012). With a content of about 0.008%, nickel (Ni) naturally occurs in the earth’s crust. Similar to Cu, Ni is a micronutrient that helps all living things perform essential tasks, but too high intakes can have adverse health effects, such as teratogenic and genotoxic effects (Demirezen and Uruc 2006; Picarelli et al. 2021). Natural mineral forms of zinc (Zn) are present. According to Tziouvalekas and Karyotis (2018) and Wolf et al. (2022), the largest single source of zinc entering the aquatic environment is the erosion of soil particles containing zinc. Zinc is a crucial trace element known to be a component of several enzyme systems in organisms. However, large quantities of zinc in aquatic ecosystem, can be toxic leading to harmful and undesirable health effects to humans (Li 2019).
The overall aim of this study is to assess if PTMs in fish tissue have increased to harmful concentrations for human consumption. In this context, we hypothesise that concentrations of PTMs in fish tissue would be significantly higher in fish landing sites near river inlets or mining sites where pollution would enter the lake. Moreover, we hypothesise that if PTMs bioaccummulate, their concentration would be higher in the predatory Clarias gariepinus.
Materials and Methods
Lake Manyara is located about 960 m above sea level. It is a shallow alkaline lake formed in a depression in the Rift Valley System of northern Tanzania (Loth and Prins 1986). The lake’s area depth varies significantly depending on the season. The lake reaches its maximum capacity during rainy season when it is 40 km long, 15 km wide, and has a depth of 3.7 m, maximum. The three major contributing rivers are the permanent Dudumera River, originating from the southern highlands, the ephemeral Makuyuni River, originating from the semi-arid savannah and highlands to the east, and the permanent Mto wa Mbu River, originating from the Ngorogoro highland to the north. Intensive irrigation agricultural activities are performed on the floodplains of the Dudumera and Mto wa Mbu rivers near their inlets. The Dudumera and Makuyuni rivers are the major sources of sediment to Lake Manyara (Wynants et al. 2020). Agriculture and mining are some of the human activities in the catchment leading to soil erosion and discharge of pollutants to the lake (Janssens de Bisthoven et al. 2020). Lake Manyara is a soda lake with no outflow and its waters are caustic and saline depending on varying freshwater input during rainy and dry seasons. The aquatic ecology of the lake is characterised by a low diversity of halophilic species, wherein the primary production is typically dominated by the cyanobacterium Arthrospira fusiformis (Kihwele et al. 2014). The lake only contains two fish species, African catfish (Clarias gariepinus) and endemic Manyara tilapia (Oreochromis amphimelas). The fish production in the main lake follows a boom and bust cycle, where productivity halts during the dry season when the waters become too saline and caustic. During these times, the fish population concentrates near the permanent freshwater river inlets, where the salinity and alkalinity is lower. During the wet season, the main lake becomes less caustic and highly productive, and the fish population rapidly booms. Oreochromis amphimelas is endemic to Lake Manyara and some other soda lakes of the Tanzanian Rift Valley. It is a maternal mouthbrooder and its population booms during the wet season when the salinity and alkalinity of the lakes goes down. In Lake Manyara, it mainly feeds on the plankton cyanobacteria and other microalgae species (Trewavas 1983). Clarias gariepinus is an omnivore and moves down from the permanent freshwater rivers and wetlands to the lake during the wet season to feed on the high number of tilapia. The lake and its surrounding ecosystem supports one of the largest colony of Lesser Flamingo (Phoeniconaias minor) and more than 390 other species of birds. During the fishing season (April to November), temporal fishing camps are set up along the northern, eastern, and southwestern shores of the lake, supporting both local households and migratory fishermen. Fishing is mostly concentrated on the eastern and southern parts and is not allowed within roughly one third of the lake that is part of the national park.
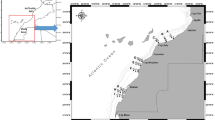
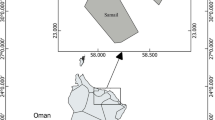
Samples of African catfish and tilapia (Clarias gariepinus and Oreochromis amphimelas respectively) were collected in April 2022 from five fish landing sites in Lake Manyara as shown in Fig. 1. All fish from the lake are unloaded at these landing sites and therefore these locations are the primary sources of fish for the local and outside markets. Ten samples of African catfish (Fig. 2A) were collected from each sampling point regardless of their size in order to represent what is available for consumption by the public. For tilapia (Fig. 2B), samples were collected by weight (9–10 kg) from each sampling point because of their small body size. Samples were kept in cool boxes and transferred to the Tanzania Atomic Energy Commission (TAEC) Laboratories in Arusha for preparation to obtain edible tissues (muscle and skin), where they were rinsed with distilled water (EPA 2000) and dissected to get to be analysed for trace elements. In this case, edible tissues from all the collected fish were kept separate per fish type and mixed into composite samples per sampling site. All utensils were cleaned according to the procedure described in (EPA 2000) using detergents, tap water, distilled water and 0.5% nitric acid prior to their use in sample preparation. Composite samples were dried in an oven at 60°C for 72 h then grounded using mortar and pestle. Grounded samples were stored in desiccators or plastic zip bags to avoid of moisture absorption as described in Sawe et al. (2019).
Sediment samples from Lake Manyara were collected using simple surface grabs of the top 5 cm on 44 locations in four different areas of the Lake (Fig. 1): northeast, northwest, southeast and southwest (Wynants et al. 2020). While a gridded approach to sediment sampling would be more appropriate (Bai et al. 2011), this was logistically not possible due to the large size of the lake and unsuitability of the sodic water for outboard engines.
Each samples stored in desiccators or plastic zip bags was divided into three portions of around 4 g. Each portion was mixed with around 0.9 g of the binding material, highly homogenized, and pressed in pellet form with a diameter of about 32 mm. The pellets were analyzed using Energy Dispersive X-Ray Fluorescence (EDXRF) spectrometry system utilizing X-Lab Pro™ software. Total elemental concentrations were estimated using the relation below (Rousseau et al. 1996).
where CE = elemental concentration (e.g. mg kg–1) of element ‘E’; KE = the calibration constant for element ‘E’ (%/kcps); IE = net peak intensity for analyte element ‘E’ (kcps) and ME = matrix correction term for element ‘E’. The calibration constant is established using a standard sample.
The analytical accuracy was verified by adding a material of known elemental concentrations (same matrix) to every set of fish samples analyzed on the EDXF. Slight deviation (s) from expected values were adjusted to ensure validity of reported results. The certified reference material (IAEA–436)—trace elements and methyl mercury in tuna fish flesh homogenate (IAEA 2006) was used during this study.
The established amounts of potentially toxic metals in fish were compared with FAO/WHO permissible levels (mg.kg−1) and FAO/WHO Provisional Table Weekly Intake (PTWI), per kg of body weight of approximately 70 kg (see Table 1). According to WHO (2021), “PTWI is an estimate of the amount of a substance in air, food, soil or drinking water that can be assimilated weekly per unit body weight (bw) over a lifetime without causing considerable health risk”.
Collected sediments were sieved to < 63 µm and analysed by Wave Length Dispersive X-Ray Fluorescence (WD-XRF, PANalytical Axios Max; OMNIAN application) as pressed pellets in the University of Plymouth Consolidated Radioisotope Facility as discussed in Wynants et al. 2020.
The data was tested for normality using Shapiro–Wilk test, where Ni and Cu were found to be non-normally distributed p ˃ 0.05 (0.09 and 0.11, respectively), while Zn and Pb were normally distributed p ˂ 0.05 (0.001 and 0.008) respectively. The non-parametric Kruskal Wallis test was carried out in the Statistical Package for Social Science (SPSS) to test if there were significant differences in the analytical concentrations measured between different sites and different species. A p-value less than 0.05 (p < 0.05) was considered statistically significant and vice versa. Sediment PTM concentrations were compared between the different lake areas to evaluate the difference in exposure levels (Bai et al. 2011).
Results and Discussion
The concentrations of potentially toxic elements in fish from Lake Manyara Northern Tanzania are presented in Tables 2 and 3. Results are reported as minimum (min), maximum (max), and mean values with their corresponding standard deviations (SD) and are presented in mg kg−1 dry weight (dw). The elements covered in this study are copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn). From Tables 2 and 3, it is evident that the maximum level of Cu (16.91 mg kg−1) was measured in African catfish sampled from Oltukai and the lowest level (5.73 mg kg−1) was measured in African catfish sampled from Minjingu. Cu levels in fish from literature have been reported as ranging from 0.7 to 27.0 mg kg–1 in muscles of fish (Tapia et al. 2006), 254 mg kg–1 in the livers of fish (Fernandes et al. 2007), 1.65–9.17 ng g–1 in muscles of fish (Mziray and Kimirei 2016). The obtained results were within the maximum allowable level of 30 mg kg–1 (WHO 1989).
African catfish samples taken at Mfulo wa Ng’ombe and Minjingu had respectively the highest (0.81 mg kg−1) and lowest (0.38 mg kg−1) concentrations of lead (Table 3). These values are within the ranges of lead concentrations for fish muscles from the Black and Aegean seas reported in the literature between 0.33 and 0.93 mg kg−1 (Uluozlu et al. 2007), 0.01 to 0.15 mg kg−1 (dw) for fish muscles from the Ria de Averio in Portugal (Perez et al. 2001), 71 to 278 μg kg−1 (dw) for fish muscles from the Eastern Aegean Sea (Uluozlu et al. 2007). The results of this study were within permissible limits, and the maximum allowed level of lead in fish is 1.50 mg kg−1 (Joint Expert Committee for Food Additives 2003), Ishak et al. 2020).
As shown in Tables 2 and 3, African catfish samples taken from Oltukai station had the highest Ni concentration (0.91 mg kg−1) and tilapia samples taken from Minjingu station had the lowest (0.62 mg kg−1). According to reports, fish muscle tissue from Indian markets have Ni concentrations between 0.03 and 1.38 mg kg−1 (Sivaperumal et al. 2007), between 0.12 and 0.15 mg kg−1 in coastal Tanzania (Mziray and Kimirei 2016), and between 0.06 and 1.59 mg kg−1 in the Mediterranean Sea (Turkmen et al. 2008). Maximum Ni concentrations in fish are not known, but the WHO has set the Provisional Table Weekly Intake (PTWI) at 2.45 mg person−1 week−1 kg of body weight−1. Basing on the highest concentration of Ni in African catfish (0.91 mg kg−1), a person could eat approximately 2.7 kg of fish muscle tissue from Manyara in a week. It would be 3.1 kg based on the overall mean in African catfish (0.80 mg kg−1). Given these high masses, it is unlikely that the Ni concentrations in fish from Lake Manyara pose a risk to human’s health.
The highest level of Zn (52.80 mg kg–1) was measured in tilapia sampled at Migunga mitatu and the lowest (33.84 mg kg–1) was measured in African catfish also sampled at Migunga mitatu (Table 3). It was interesting to note that the highest and lowest levels of Zn were measured at the same location and in different fish species, especially considering that the average value of Zn is higher in African catfish than tilapia. The reason for this observation is not immediately clear. Levels of Zn reported for fish in other parts of the world are in the range of 1.35–6.69 mg kg−1 in muscle, 2.71–78.70 mg kg−1 in liver, and 7.27–16.87 mg kg−1 in gonads (Uluturhan and Kucuksezgin 2007). The obtained results were within the maximum allowable levels of Zn which is 100 mg kg–1 for fish (Nauen 1983, WHO 1989).
In this study, the overall mean concentrations of metals in both fish species together (not shown in the tables) were found to be 41.18, 10.87, 0.80 and 0.62 mgkg−1 for Zn, Cu, Ni and Pb, respectively. This suggest a trend of Zn > Cu > Ni > Pb for the two fish species combined. Looking at the species separately, tilapia had average concentrations of 0.79, 11.29, 40.13 and 0.64 mg kg−1 for Ni, Cu, Zn, and Pb, respectively (Table 2). African catfish showed an average concentration of 0.80, 10.45, 42.22 and 0.60 mgkg−1 for nickel, copper, zinc and lead, respectively (Table 3). The elemental concentrations in both tilapia and African catfish followed the trend of Zn > Cu > Ni > Pb. This trend suggest essential elements had higher concentrations than the non-essential element (Pb). The trend may endorse the essential biological roles of Zn, Cu and Ni in fish (Chen and Chen 2001, Bahnasawy et al. 2009). The results further showed that the concentrations of Cu and Pb were slightly higher in tilapia, while Ni and Zn were slightly higher in African catfish. These slight differences could be explained due to the specific feeding behaviour or differences in metabolic pathways. Evidenced by the small standard deviation, the variation in PTM concentration within the species was relatively low. The remaining variance can be explained by differences in life stage, size of the fish, and catchment characteristics of the dominant contributing tributary. This study focused on fish available in the market regardless of their size. However, further research on differentiating PTM concentrations in different fish sizes and organs is desirable since it could form an additional indication if metals are accumulating when fish are growing or in certain parts of fish.
Variations of elemental concentrations between fish types investigated by Kruskal–Wallis test revealed that there were no significant differences in Nickel (p = 0.40), Lead (p = 0.40), Copper (p = 0.30) and Zinc (p = 0.34) concentrations across categories of tilapia and African catfish. Since no significant higher values of metals were found in the omnivorous African catfish, we can infer that the investigated PTMs are not accumulating higher up the food chain in Lake Manyara, allowing us to reject this hypothesis. Moreover, there was no difference in the PTM variance within the species, which was low in both species. Since the fish population in Lake Manyara supports a large number and diversity of fish-eating avifauna (Janssens de Bisthoven et al. 2020), it is unlikely these are negatively impacted by the studied potential toxic metals. However, further investigation of PTM concentrations in the fish-eating avifauna, suspended materials, and in the liver and kidneys of fish is needed to validate this finding. Moreover, a better understanding of the Lake Manyara foodweb is needed for a holistic overview of the potential impacts of PTMs on the entire ecosystem (Lemmens et al. 2017).
The analysis of PTMs in the sediment samples (Table 4) revealed significant differences in their concentrations between different areas in the lake. The concentration of Ni in the sediments varied significantly (p < 0.01), with higher values in the north. Sediment Pb also varied significantly (p < 0.01) with highest concentrations in the southwest. Zn concentrations in the sediments also significantly differed (p-value < 0.01) with the highest values in the northeast. Only the Cu concentrations did not significantly differ in lake sediments (p = 0.32). However, it is important to note the high variance in Cu concentration driven by some high outlier values. These high differences in PTM concentrations in the sediments are most likely caused by natural differences in the geochemistry of the contributing tributaries (Wynants et al. 2020), although unnaturally high Cu concentrations have been observed in soils under coffee plantations located in the Makuyuni catchment, most likely due to fungicide applications (Wynants et al. 2021a). However, regardless of these differences in sediment chemistry, there were no significant differences in the fish muscle concentrations of Nickel (p = 0.29), Copper (p = 0.29), Zinc (p = 0.33), and Lead (p = 0.35) between the different landing sites. If increased mobilisation of metals from mining or erosion in the wider catchment would cause increased uptake of PTMs in fish, we would expect these concentrations to be higher in the fish landing sites near the main river inlets and the hotspot PTM concentration areas (Bai et al. 2011). The lack of significant differences between the fish landing sites indicate that we can reject this hypothesis. The concentration of PTMs in the fish were also an order of magnitude lower compared to those in the sediments, indicating that they are not impacted by PTMs entering the lake as sediments. This is likely because the PTMs remain bound to the sediment particles and are relatively immobile in the alkaline environment of Lake Manyara (Kicińska et al. 2022). However, additional analyses are needed on the water chemistry of Lake Manyara to gain a better understanding of PTM mobility in its total environment. In this study, the fish were gathered in April nearing the end of the rainy season. However, it is possible that when the lake dries, the concentration of PTMs will increase, potentially affecting the concentrations in the fish tissues. A promising area of further study is to monitor potential increases in PTM concentrations throughout the fishing season.
Lack of fish consumption data of the human population in the study area and in Tanzania as a whole prevented the assessment of PTM intake due to fish consumption. However, using the WHO’s Provisional Table Weekly Intake (PTWI) of metals, we developed the first recommendation of the amount of Lake Manyara fish that can be consumed without appreciable health risk. Based on the highest concentration of each metal found in fish, one is recommended to consume a maximum of 14.5, 2.7, 2.2, and 9.3 kg of fish per week in order to reach the PTWI for Cu, Ni, Pb and Zn respectively. Since the 2.2 kg related to Pb concentration is the lowest number, this could act as a provisional guideline for fish consumption from Lake Manyara.
Levels of lead (Pb), zinc (Zn), copper (Cu) and nickel (Ni) in edible parts of tilapia and African catfish from Lake Manyara Northern Tanzania were assessed during this study. The highest levels of Nickel and copper were found in African catfish at the Oltukai site, while the highest levels of zinc were found in tilapia at Migunga mitatu site. The highest lead concentration was found in African catfish at Mfulo wa ng’ombe site. The results did not show any significant differences in PTM concentrations between Manyara tilapia and African catfish, nor between sampling points. This indicates that the metals are not accumulating in the food chain, nor are the fish near the river inlets more impacted, allowing us to reject these hypotheses. The trend of elemental concentrations in both tilapia and African catfish followed Zn > Cu > Ni > Pb and none of the analyzed elements exceeded the permissible levels recommended by the Food and Agriculture Organization of the United Nations (FAO). This indicates that the lake and the fish are not impacted by pollution of the studied metals.
Based on the Provisional Table Weekly Intake of metals of the World Health Organization (WHO) and the highest found concentration of Pb in fish, we recommend a maximum consumption of 2.2 kg fish muscle tissue per week from Lake Manyara. Given this relatively high value, these results suggest that it is unlikely that the analyzed PTMs form a health risk to the fish consumers in Tanzania. Based on the results of this study, it can be concluded that fish from Lake Manyara are safe for human consumption provided that the amount consumed does not exceed estimated values based on PTWI. Nonetheless, continued monitoring of heavy metals in fish from Lake Manyara is recommended because of the increasing human activities in the catchment area.
References
Bahnasawy MKA (2009) Seasonal variations of heavy metals concentrations in mullet, Mugil cephalus and Liza ramada (Mugilidae) from Lake Manzala. Egypt J Aquat Biol Fisher 1:81–100. https://doi.org/10.21608/EJABF.2009.2034
Bai J, Cui B, Chen B, Zhang K, Deng W, Gao H, Xiao R (2011) Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecol Model 222:301–306. https://doi.org/10.1016/j.ecolmodel.2009.12.002
Banzi FP, Kifanga LD, Bundala FM (2000) Natural radioactivity and radiation exposure at the Minjingu phosphate mine in Tanzania. J Radiol Prot 20:41. https://doi.org/10.1088/0952-4746/20/1/305
Chen CY, Chen MH (2001) Heavy metal concentrations in nine species of fishes caught in coastal waters off Ann-Ping, SW Taiwan. J Food Drug Anal 9:107–114. https://doi.org/10.38212/2224-6614.2803
D’Souza H, Sebastian A, Geraldine M, Venkatesh T (2011) Diagnosis, treatment of lead poisoning in general population. Ind J Clin Biochem 26:197–201. https://doi.org/10.1007/s12291-011-0122-6
Demirezen KUD, Uruc K (2006) Comparative study of trace elements in certain fish, meat and meat products. Meat Sci 74:255–260. https://doi.org/10.1016/j.meatsci.2006.03.012
EPA (2000) Guidance for assessing chemical contaminant data for use in fish advisories, Volume 1: fish sampling and analysis (Third Edition). Office of Water, EPA 823-B-00.
Erkkilä AT, Lichtenstein AH, Mozaffarian D, Herrington DM (2004) Fish intake is associated with a reduced progression of coronary artery atherosclerosis in postmenopausal women with coronary artery disease. Am J Clin Nutr 80:626–632. https://doi.org/10.1093/ajcn/80.3.626
FAO (2020) The state of world fisheries and aquaculture. Sustainability in action, Rome. https://doi.org/10.4060/ca9229en
FAO (2018) The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals
Fernandes C, Fontainhas-Fernandes A, Peixoto F, Salgado MA (2007) Bioaccumulation of heavy metals in Liza saliens from the Esmoriz-Paramos coastal lagoon, Portugal. Ecotoxicol Environ Saf 66:426–431. https://doi.org/10.1016/j.ecoenv.2006.02.007
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58. https://doi.org/10.2478/v10102-012-0009-2
IAEA (2006) Trace elements and methyl mercury in tuna fish flesh homogenate. IAEA, Vienna
Ishak AR, Zuhdi MSM, Aziz MY (2020) Determination of lead and cadmium in tilapia fish (Oreochromis niloticus) from selected areas in Kuala Lumpur. Egypt J Aquat Res 46:221–225. https://doi.org/10.1016/j.ejar.2020.06.001
Ivo S, Ralf D, Julian FB (2013) Copper: effects of deficiency and overload. In: Sigel A, Sigel H, Sigel RKO (eds) Interrelations between essential metal ions and human diseases. Metal ions in life sciences, vol 13. Springer, Dordrecht, pp 359–387. https://doi.org/10.1007/978-94-007-75.2013
Janssens de Bisthoven L, Vanhove MPM, Rochette AJ et al (2020) Social-ecological assessment of Lake Manyara basin, Tanzania: a mixed method approach. J Environ Manage 267:110594. https://doi.org/10.1016/j.jenvm
Joint Expert Committee for Food Additives (2003) Summary and Conclusions of the 61st Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). JECFA/61/SC. Rome, Italy.
Kihwele ES, Lugomela C, Howell KM (2014) Temporal changes in the Lesser Flamingos population (Phoenicopterus minor) in relation to phytoplankton abundance in Lake Manyara, Tanzania. Open J Ecol 4:145–161. https://doi.org/10.4236/oje.2014.43016
Kicińska A, Pomykała R, Izquierdo-Diaz M (2022) Changes in soil pH and mobility of heavy metals in contaminated soils. Eur J Soil Sci 73:e13203. https://doi.org/10.1111/ejss.13203
Klaverkamp JF, MacDonald WA, Duncan DA, Wagemann R (1984) Metallothionein and accumulation to heavy metals in fish: a review. In: Cairns VW, Hodson PV, Nriagu JO (eds) Contaminant effects on fisheries. Wiley, New York
Lee S, Roesel D, Roke S (2021) Imaging Cu2+ binding to charged phospholipid membranes by high-throughput second harmonic wide-field microscopy. J Chem Phys 155:184704
Lemmens P, Teffera FE, Wynants M, Gorvaert L, Deckers J, Bauer H, De Meester LT (2017) Intra- and interspecific niche variation as reconstructed from stable isotopes in two ecologically different Ethiopian Rift Valley lakes. Funct Ecol 31:1482–1492. https://doi.org/10.1111/1365-2435.12852
Li X, Wang PF, Feng CL et al (2019) Acute toxicity and hazardous concentrations of zinc to native freshwater organisms under different pH values in China. Bull Environ Contam Toxicol 103:120–126. https://doi.org/10.1007/s00128-018-2441-2
Loth PE, Prins HH (1986) Spatial patterns of the landscape and vegetation of Lake Manyara National Park. ITC Journal 1:115–130
Mărginean C, Meliţ L, Moldovan H, Lupu V, Mărginean M (2016) Lead poisoning in a 16-year-old girl: a case report and a review of the literature (CARE compliant). Medicine 95:e4916. https://doi.org/10.1097/MD.0000000000004916
Mng’ong’o M, Munishi LK, Ndakidemi PA, Blake W, Comber S, Hutchinson TH (2021) Toxic metals in East African agro-ecosystems: key risks for sustainable food production. J Environ Manage 294:112973. https://doi.org/10.1016/j.jenvman.2021
Mziray P, Kimirei I (2016) Bioaccumulation of heavy metals in marine fishes (Siganus sutor, Lethrinus harak, and Rastrelliger kanagurta) from Dar es Salaam Tanzania. Reg Stud Mar Sci 7:72–80. https://doi.org/10.1016/j.rsma.2016.05.014
Nauen CE (1983) Compilation of legal limits of hazardous substances in fish and fishery products. FAO Fish Circular 764:102
Nonga HE, Sandvik M, Miles CO et al (2015) Possible involvement of microcystins in the unexplained mass mortalities of Lesser Flamingo (Phoeniconaias minor Geoffroy) at Lake Manyara in Tanzania. Hydrobiologia 678:167–178. https://doi.org/10.1007/s10750-011-0844-8
Perez Cid B, Boia C, Pombo L, Robelo E (2001) Determination of trace metals in fish species of the Ria de Aveiro (Portugal) by electrothermal atomic absorption spectrometry. Food Chem 75:93–100. https://doi.org/10.1016/S0308-8146(01)00184-4
Picarelli A, Greco N, Sciuttini F, Marini C, Meacci A (2021) High consumption of Nickel-containing foods and IBS-like disorders: late events in a gluten-free diet. Ecotoxicol Environ Saf 222:112492. https://doi.org/10.1016/j.ec
Rousseau MR, Willis PJ, Dunkan RA (1996) Practical XRF calibration procedures for major and trace elements. X-Ray Spectrom 25:179–189. https://doi.org/10.1002/(SICI)1097-4539(199607)25:4%3c179
Sawe SF, Shilla DA, Machiwa JF (2019) Assessment of enrichment, geo-accumulation and ecological risk of heavy metals in surface sediments of the Msimbazi mangrove ecosystem, coast of Dar es Salaam, Tanzania. Chem Ecol 35:835–845. https://doi.org/10.1080/02757540.2019.1663344
Shechonge A, Ngatunga BP, Bradbeer SJ et al (2019) Widespread colonisation of Tanzanian catchments by introduced Oreochromis tilapia fishes: the legacy from decades of deliberate introduction. Hydrobiologia 832:235–253. https://doi.org/10.1007/s10750-018-3597-9
Sivaperumal P, Sankar TV, Nair PV (2007). Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food chem 102:612-620. https://doi.org/10.1016/j.foodchem.2006.05.041
Solgi E, Alipour H, Majnooni F (2019) Investigation of the concentration of metals in two economically important fish species from the Caspian sea and assessment of potential risk to human health. Ocean Sci J 54:503–514. https://doi.org/10.1007/s12601-019-0024-8
Tam NFY, Wong YS (2000) Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ Pollut 110:195–205. https://doi.org/10.1016/S0269-7491(99)00310-3
Tapia J, Duran E, Pena-Cortes F et al (2006) Micropogonias as a bioindicator for copper in Lake Budi (IX Region, Chile). J Chilean Chem Soc 51:901–904. https://doi.org/10.4067/S0717-97072006000200013
Trewavas E (1983) Tilapiine fishes of the genera Sarotherodon, Oreochromis and Danakilia. British Museum, London, p 583
Turkmen M, Turkmen A, Tepe Y et al (2008) Determination of metal contaminations in sea foods from Marmara, Aegean and Mediterranean seas: twelve fish species. Food Chem 108:794–800. https://doi.org/10.1016/j.foodchem.2007.11.025
Tziouvalekas M, Karyotis T (2018) Zinc in soils, water and food crops. J Trace Elem Med Biol 49:252–260. https://doi.org/10.1016/j.jtemb.2018.02.009
Uluozlu OD, Tuzen M, Medil D, Soylak M (2007) Trace metal content in nine species of fish from the Black and Aegean Seas, Turkey. Food Chem 104:835–840. https://doi.org/10.1016/j.foodchem.2007.01.003
Uluturhan E, Kucuksezgin F (2007) Heavy metals contamination in Red Pandora (Pagellus erythrinus) tissues from the eastern Aegean Sea, Turkey. Water Res 4:1185–1192. https://doi.org/10.1016/j.watres.2006.11.044
URT (2010) Fisheries Sector Development Programme 2010. Ministry of livestock and fisheries development.
World Health Organization (1989). Heavy metals-environmental aspects. Environment Health Criteria No. 85. WHO, Geneva
World Health Organization (2021) Human health risk assessment toolkit: chemical hazards, 2nd edn. WHO, Geneva
Wolf J, Sandstead HH, Rink L (2022) Chapter 38 – Zinc. In: Nordberg GF, Costa M (eds) Handbook on the toxicology of metals, specific metals, vol 2. Academic Press, London, pp 963–980
Wynants M, Millward G, Patrick A et al (2020) Determining tributary sources of increased sedimentation in East-African Rift Lakes. Sci Total Environ 717:137266. https://doi.org/10.1016/j.scitotenv.2020.137266
Wynants M, Solomon H, Ndakidemi P, Blake WH (2018). Pinpointing areas of increased soil erosion risk following land cover change in the Lake Manyara catchment, Tanzania. Int J Appl Earth Obs Geoinf 71. https://doi.org/10.1016/j.jag.2018.05.008
Wynants M, Patrick A, Munishi L, Mtei K, Bodé S, Taylor A, Millward G, Roberts N, Gilvear D, Ndakidemi P, Boeckx P, Blake WH (2021a) Soil erosion and sediment transport in Tanzania: Part I – sediment source tracing in three neighbouring river catchments. Earth Surf Process Landf 46:3096–3111. https://doi.org/10.1002/esp.5217
Wynants M, Patrick A, Munishi L, Mtei K, Bodé S, Taylor A, Millward G, Roberts N, Gilvear D, Ndakidemi P, Boeckx P, Blake WH (2021b) Soil erosion and sediment transport in Tanzania: Part II – sedimentological evidence of phased land degradation. Earth Surf Process Landf 46:3112–3126. https://doi.org/10.1002/esp.5218
Acknowledgements
The author acknowledges financial and technical support from the Tanzania Atomic Energy Commission.
Funding
Open access funding provided by Swedish University of Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest associated with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sawe, S., Amasi, A. & Wynants, M. Assessment of Potentially Toxic Metals in Fish from Lake Manyara, Northern Tanzania. Bull Environ Contam Toxicol 111, 39 (2023). https://doi.org/10.1007/s00128-023-03794-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-023-03794-6