Abstract
Aims/hypothesis
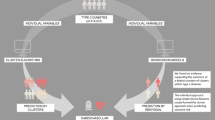
Type 2 diabetes is a heterogeneous disease process with variable trajectories of CVD risk. We aimed to evaluate four phenomapping strategies and their ability to stratify CVD risk in individuals with type 2 diabetes and to identify subgroups who may benefit from specific therapies.
Methods
Participants with type 2 diabetes and free of baseline CVD in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial were included in this study (N = 6466). Clustering using Gaussian mixture models, latent class analysis, finite mixture models (FMMs) and principal component analysis was compared. Clustering variables included demographics, medical and social history, laboratory values and diabetes complications. The interaction between the phenogroup and intensive glycaemic, combination lipid and intensive BP therapy for the risk of the primary outcome (composite of fatal myocardial infarction, non-fatal myocardial infarction or unstable angina) was evaluated using adjusted Cox models. The phenomapping strategies were independently assessed in an external validation cohort (Look Action for Health in Diabetes [Look AHEAD] trial: n = 4211; and Bypass Angioplasty Revascularisation Investigation 2 Diabetes [BARI 2D] trial: n = 1495).
Results
Over 9.1 years of follow-up, 789 (12.2%) participants had a primary outcome event. FMM phenomapping with three phenogroups was the best-performing clustering strategy in both the derivation and validation cohorts as determined by Bayesian information criterion, Dunn index and improvement in model discrimination. Phenogroup 1 (n = 663, 10.3%) had the highest burden of comorbidities and diabetes complications, phenogroup 2 (n = 2388, 36.9%) had an intermediate comorbidity burden and lowest diabetes complications, and phenogroup 3 (n = 3415, 52.8%) had the fewest comorbidities and intermediate burden of diabetes complications. Significant interactions were observed between phenogroups and treatment interventions including intensive glycaemic control (p-interaction = 0.042) and combination lipid therapy (p-interaction < 0.001) in the ACCORD, intensive lifestyle intervention (p-interaction = 0.002) in the Look AHEAD and early coronary revascularisation (p-interaction = 0.003) in the BARI 2D trial cohorts for the risk of the primary composite outcome. Favourable reduction in the risk of the primary composite outcome with these interventions was noted in low-risk participants of phenogroup 3 but not in other phenogroups. Compared with phenogroup 3, phenogroup 1 participants were more likely to have severe/symptomatic hypoglycaemic events and medication non-adherence on follow-up in the ACCORD and Look AHEAD trial cohorts.
Conclusions/interpretation
Clustering using FMMs was the optimal phenomapping strategy to identify replicable subgroups of patients with type 2 diabetes with distinct clinical characteristics, CVD risk and response to therapies.
Graphical abstract

Similar content being viewed by others

Introduction
The prevalence of type 2 diabetes is increasing with the rising burden of obesity and sedentary lifestyle [1]. Among patients with type 2 diabetes, CVD is the leading cause of morbidity and mortality, highlighting the need for implementation of effective cardiovascular preventive strategies that may match the intensity of therapy to the baseline risk [2]. A critical next step for CVD prevention is to identify individuals with type 2 diabetes who have heightened but modifiable risk for adverse cardiovascular events and may derive the greatest benefit from initiating or intensifying disease-modifying therapies, such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists [3, 4]. Accordingly, recent studies have developed clinical risk scores to identify those who are at the highest risk for adverse cardiovascular events among cohorts of individuals with type 2 diabetes [5,6,7]. However, these traditional risk assessment models do not account for multidimensional data and do not differentiate between modifiable vs non-modifiable risk.
Prior studies have utilised machine learning to identify clusters of patients with traditional risk factors, like primary hypertension [8], and subgroups of patients with prevalent CVD, including CHD [9] and heart failure [10,11,12]. While clustering may identify clinically unique subtypes in a given population, unsupervised machine learning may identify subtypes that are unrelated to patient outcomes. Conversely, supervised machine learning may yield subgroups that are not biologically meaningful. Few studies have characterised the benefits and shortcomings of different clustering strategies to identify type 2 diabetes patient phenotypes. The aim of the present study was to compare semi-supervised clustering methodologies to identify phenotypic subgroups within a cohort of individuals with type 2 diabetes at high CVD risk enrolled in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial [13]. For this, we evaluated a novel semi-supervised methodology in addition to several clustering techniques that have been previously studied [14]. Subsequently, the phenogroups derived from the optimal clustering strategy were externally validated in two separate cohorts of patients with type 2 diabetes from the Look Action for Health in Diabetes (Look AHEAD) and Bypass Angioplasty Revascularisation Investigation 2 Diabetes (BARI 2D) trials [15, 16].
Methods
Study populations
The derivation cohort comprised participants from the ACCORD trial and its long-term observational follow-up study (ACCORD Follow-on [ACCORDION]) [13, 17, 18]. A detailed description of the ACCORD trial is provided in the electronic supplementary material (ESM) Methods. Participants with a history of CVD (i.e. previous stroke or CHD) or >10% missing data were excluded. External validation of the phenomapping approach was performed in participants from the Look AHEAD trial. The prognostic usefulness in identifying patients who will benefit from type 2 diabetes therapies was also assessed in the ACCORD and Look AHEAD cohorts. Furthermore, to assess the generalisability of the phenomapping approach in a higher-risk cohort, we additionally evaluated the prognostic significance of the phenogroups and their interaction with revascularisation among participants of the BARI 2D trial who had type 2 diabetes and coronary artery disease. The study design and results from the Look AHEAD and BARI 2D trials have been published previously and descriptions are provided in the ESM Methods [15, 16, 19]. All participants in ACCORD, Look AHEAD and BARI 2D had type 2 diabetes at baseline.
Outcomes of interest
The primary outcome of interest for the present study was a major CHD event defined as a composite of fatal myocardial infarction (MI), non-fatal MI or unstable angina. Each component of the primary outcome was evaluated as a secondary outcome and adjudicated by a central committee masked to randomised assignments in ACCORD. In the external validation cohorts, the primary outcome included a composite of fatal or non-fatal MI in BARI 2D with the addition of hospitalisation for angina in Look AHEAD. Descriptions of the outcomes evaluated in each of the three trial cohorts are included in the ESM Methods [13, 15, 16]. The secondary outcomes of interest for the present study were all-cause mortality, cumulative severe/symptomatic hypoglycaemic (SH) events and treatment non-adherence. A detailed description of the secondary outcomes is provided in the ESM Methods.
Phenotypic variable selection
The detailed variable selection and clustering methodologies are described in the ESM Methods and outlined in ESM Fig. 1. A total of 97 candidate variables were considered for inclusion. After excluding covariates with >10% missingness, high correlation (|r| > 0.70), or composites of already present covariates a total of 44 continuous and categorical variables were included in the final analysis. Prior to clustering, missing data (not including the outcome variable) were singularly imputed [20].
The Wald index was used to identify relevant covariates [21]. The Wald index is a hypothesis test of statistical significance and is analogous to the Cox score (a measure of the correlation between the covariate and outcome of interest). The Wald index ranges from zero to infinity and a higher value indicates a stronger association between the covariate and outcome. The Wald index for each covariate was calculated and ranked. Due to the limited performance increase in clustering more than 20 covariates, only variables with the highest 20 Wald indices were included [11].
Clustering methodologies
After identifying the 20 covariates to be included in the analyses, continuous variables were scaled and standardised (mean = 0; standard deviation = 1). Four clustering methodologies were evaluated: Gaussian mixture model (GMM)-based clustering of continuous variables, latent class analysis (LCA) of categorical and binned continuous variables, finite mixture model (FMM)-based clustering of both continuous and categorical (mixed) data, and dichotomisation of the principal component (principal component analysis [PCA]) of mixed data into two clusters. An explanation of each method is detailed in the ESM Methods. For each phenomapping method, the Bayesian information criterion (BIC) and Dunn index were calculated to determine the optimal number of clusters (evaluated two to seven, except for PCA) [22, 23].
Optimal clustering method
The optimal clustering method was defined according to the best-performing BIC (lower values considered better), Dunn index (higher values considered better) and prognostic utility. The prognostic value of the phenogroup membership was determined by evaluating the improvement in performance in the 2013 pooled cohort equation (PCE) with vs without the phenogroup variable [24]. Improvement in mean positive predictive value (PPV) was used to assess the comparative utility of PCE-only and PCE + phenogroup [25]. The different clustering methodologies were also independently compared in the Look AHEAD trial validation cohort.
Once identified, we further assessed the prognostic utility of the optimal clustering method by evaluating the improvement in mean PPV with the Swedish National Diabetes Register (SNDR) score with and without phenogroup membership [26]. Finally, decision curves, a measure of the number of true positive cases identified without an increase in the false-positive rate, were constructed to compare phenogroup ± PCE and phenogroup ± SNDR scores [27].
Statistical analysis
In the derivation cohort, after identification of the optimal clustering method and respective clusters, phenogroup characteristics were compared across the subgroups. Categorical variables were summarised as percentages and differences across phenogroups were evaluated using the χ2 test. Continuous variables were summarised as means (standard deviations) and differences across phenogroups were evaluated using one-way ANOVA. Time-to-event analyses and logrank tests were performed to evaluate and compare the unadjusted risk of the primary outcome across phenogroups. Multiplicative interaction terms were included in multivariable-adjusted Cox models to evaluate whether phenogroup modifies the association between the treatment group and risk of the primary outcome with adjustment for the following covariates: age, sex, race, education level, treatment arm, BMI, systolic BP, alcohol use, smoking, total cholesterol, serum creatinine, high-density lipoprotein cholesterol and diabetes duration. The cumulative incidence of all-cause mortality across phenogroups was also assessed. For SH event and glycaemic medication non-adherence outcomes, one-way ANOVA and negative binomial regression were used to evaluate for differences in the cumulative number of events across phenogroups with the same model adjustments described above. To further assess the robustness of the primary analysis results, multiple sensitivity analyses were performed as outlined in ESM Methods.
In the external validation cohort of the Look AHEAD trial, we predicted which phenogroup each individual most closely resembled using multinomial logistic regression (MLR). Agreement between the MLR-predicted phenogroups and FMM phenomapping in the external validation cohort alone was determined by the Cohen’s κ statistic [28]. In the higher-risk cohort of type 2 diabetes patients from the BARI 2D trial, a similar MLR approach was also used to stratify the participants across the three phenogroups. The differences between predicted phenogroups with respect to baseline characteristics, risk of primary and secondary outcomes, and treatment interactions were analysed in both the validation cohorts similarly to the primary analysis. Analyses were performed with R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) with p < 0.05 used as the criterion for statistical significance.
Ethics approval
All participants provided written and informed consent and the study was approved by the ethics committee at each participating trial site. The present study was considered exempt by the Institutional Review Board at the University of Texas Southwestern Medical Center, Dallas, Texas.
Results
In the ACCORD trial, there were 6537 (63.8%) participants without CVD at baseline of which 6466 (63.1%) had <10% missing data and were included in the analyses. A detailed description of the covariates and inclusion/exclusion criteria is provided in ESM Table 1. A total of 44 variables were included in the semi-supervised clustering procedure to identify the top 20 most significant variables to be used in the clustering analysis (Fig. 1). The validation analysis included 4211 of 4906 (85.8%) participants with available follow-up data and no prior CVD who were enrolled in the Look AHEAD trial and 1495 of 2368 (63.1%) participants enrolled in the BARI 2D trial. Baseline characteristics of the derivation and validation cohorts are provided in ESM Table 2.
Determination of the optimal phenomapping strategy and development of a phenogroup classifier
When comparing the four phenomapping methods, clustering with FMMs and three phenogroups had the best-performing internal validation metrics including lowest BIC and highest Dunn index (ESM Table 3, ESM Fig. 2). When phenogroup membership was added to the PCE risk score, the FMM method with three clusters had the greatest improvement in model discrimination (ESM Table 3). Similar to the derivation cohort, the FMM method was the top-performing phenomapping method in the external validation cohort (Look AHEAD) (ESM Table 4).
Using the FMM phenogroups derived in the derivation cohort, we developed an MLR classifier to predict phenogroup membership for individuals (ESM Table 5). Using the MLR classifier, each participant in the validation cohort was successfully matched into one of the three previously defined clusters. We observed high agreement between the MLR classifier and phenomapping in the Look AHEAD dataset (Cohen’s κ = 0.91 [95% CI 0.88, 0.93]) and a minimum of ten non-missing covariates was needed to achieve an 80% accurate prediction in the MLR classifier (ESM Fig. 3).
Prognostic utility of the FMM phenogroups in improving atherosclerotic cardiovascular disease risk prediction
Over 9.1 years of follow-up, 789 (12.2%) and 963 (14.9%) participants had a primary outcome and all-cause mortality event, respectively. Phenogroup membership from the FMM clustering method was significantly associated with risk of the primary composite outcome and all-cause mortality on follow-up, with a graded decrease in risk from phenogroup 1 (highest risk) to phenogroup 3 (lowest risk) (ESM Fig. 4). The mean PPV of the SNDR risk score was 0.16 (95% CI 0.14, 0.19). Addition of the FMM phenogroup significantly increased the mean PPV (PPV = 0.21 [95% CI 0.20, 0.23]). Similarly, in decision curve analysis, phenogroup membership also improved the prognostic utility of both risk scores (ESM Fig. 5). In the Look AHEAD external validation cohort, addition of the FMM phenogroup to the SNDR risk score significantly increased the mean PPV (increase from 0.14 [0.12, 1.17] to 0.16 [0.14, 0.0.19]). A similar improvement was observed in decision curve analysis (ESM Fig. 5).
Characterisation of FMM-based phenogroups
Baseline characteristics of study participants across the three phenogroups as determined by the FMM method are shown in Tables 1 and 2. Compared with the other groups, phenogroup 1 participants (n = 663, 10.3%) were more likely to be men, to be of self-reported black race and to have higher burden of traditional cardiovascular risk factors, including higher BP, smoking prevalence, HbA1c, fasting blood glucose, low-density lipoprotein cholesterol and triacylglycerol levels. Phenogroup 2 (n = 2388, 36.9%) had participants with intermediate burden of CVD risk factors. In contrast, phenogroup 3 (n = 3415, 52.8%) participants had the lowest burden of CVD risk factors, with lower BP, low-density lipoprotein cholesterol levels, HbA1c and smoking prevalence. The prevalence of type 2 diabetes-related complications such as foot ulceration, lower extremity amputations, proteinuria and eye surgery history was highest in phenogroup 1 and lowest in phenogroup 2 members. The pattern of baseline characteristics was similar when phenomapping separately in men and women (ESM Table 6). The pattern of baseline characteristics across the FMM-based phenogroups in the Look AHEAD and BARI 2D validation cohorts was mostly similar to that observed in the derivation cohort (ESM Tables 7, 8).
Effect modification by FMM-based phenogroups for cardiovascular benefits associated with different therapies in type 2 diabetes
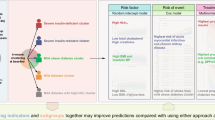
The proportion of participants randomised to intensive glycaemic control was similar across the three phenogroups in the derivation cohort (Table 1). We observed a significant interaction between randomisation to intensive glycaemic control (vs standard) and phenogroup for the risk of the primary composite outcome (p-interaction = 0.042) (Fig. 2). Intensive glycaemic control was associated with lower risk of the primary composite outcome in phenogroup 3 (adjusted HR [aHR] 0.65; 95% CI 0.51, 0.83; p value <0.001) and there was no significant association with risk in phenogroup 1 (aHR 1.25; 95% CI 0.91, 1.77; p value = 0.19). A similar pattern of association was noted with randomisation to combination lipid therapy with a significant treatment interaction by phenogroup membership (p-interaction < 0.001). Specifically, combination lipid therapy was associated with significantly lower risk in phenogroup 3 (aHR 0.71; 95% CI 0.52, 0.98; p value = 0.04) and higher risk in phenogroup 1 (aHR 1.49; 95% CI 0.98, 2.24; p value = 0.06). There was no significant treatment effect of intensive BP control on risk of the primary composite outcome across the phenogroups (Fig. 2). Event rates and number of events prevented across treatment groups and phenogroups are shown in ESM Table 9. Among participants in phenogroup 1, the risk of all-cause mortality was numerically higher in the intensive vs standard glycaemic control groups (28.5% vs 25.8%, respectively; p value = 0.34). In contrast, the risk of all-cause mortality was lower in the intensive vs standard glycaemic control group in phenogroup 3 (8.9% vs 10.9%, respectively; p value = 0.05) (ESM Fig. 6).
Multivariable-adjusted associations of randomised treatment groups with risk of primary outcome events stratified by FMM-based phenogroups in the derivation (ACCORD) and validation (Look AHEAD and BARI 2D) cohorts. Cox models were adjusted for age, sex, race, education level, BMI, systolic BP, smoking status, alcohol use, serum creatinine, total cholesterol, serum HDL-cholesterol and diabetes duration. The primary outcome was a composite of fatal MI, non-fatal MI or unstable angina in the ACCORD trial cohort. In the Look AHEAD trial cohort, the primary outcome included fatal or non-fatal MI plus hospitalisation for angina. In BARI 2D, the primary outcome was a composite of fatal MI or non-fatal MI
In the Look AHEAD external validation cohort, we observed a significant interaction between intensive lifestyle intervention (ILI) therapy (vs standard) and phenogroup for the risk of the primary composite outcome (p-interaction = 0.002) (Fig. 2). Specifically, ILI was associated with a lower risk of the primary composite outcome in phenogroup 3 (aHR 0.77; 95% CI 0.61, 0.98; p value = 0.03) and higher risk in phenogroup 1 (aHR 1.58; 95% CI 1.10, 2.47; p value = 0.03).
In the BARI 2D external validation cohort, we similarly observed a significant interaction between early coronary revascularisation (vs medical therapy) and phenogroup for the risk of the primary composite outcome (p-interaction = 0.003) (Fig. 2). Early revascularisation was associated with lower risk of the primary composite outcome in phenogroup 3 (aHR 0.64; 95% CI 0.47, 0.91; p value = 0.008) and higher risk in phenogroup 1 (aHR 1.90; 95% CI 1.07, 3.35; p value = 0.03).
Association of FMM-based phenogroups with SH events
In the derivation cohort (ACCORD trial), there were a total of 1478 SH events over a median follow-up of 8.8 (IQR = 5.7–10.1) years. The mean number of cumulative SH events per participant was highest in phenogroup 1 (0.32 events) followed by phenogroup 2 (0.26 events) and phenogroup 3 (0.19 events) (p value = 0.0004) (Fig. 3a). In adjusted analysis, the incidence rate ratio of an SH event was significantly higher in phenogroups 1 and 2 as compared with phenogroup 3 (phenogroup 1: 0.41; 95% CI 0.13, 0.69; p value = 0.005; and phenogroup 2: 0.22; 95% CI 0.04, 0.41; p value = 0.03). There was no significant interaction between phenogroup membership and intensive glycaemic control for the risk of SH events (p-interaction = 0.68). Additional adjustment for baseline glucose-lowering medications did not attenuate the associations between phenogroups and SH events.
In the Look AHEAD validation cohort, there were a total of 637 SH events over a median follow-up of 9.5 (IQR = 8.8–10.3) years. Similar to the derivation cohort, the mean cumulative number of SH events per participant was highest in phenogroup 1 (0.21 events) followed by phenogroup 2 (0.17 events) and phenogroup 3 (0.15 events) (p value = 0.03). In adjusted analysis, the incidence ratio of an SH event was significantly higher in phenogroup 1 (0.33; 95% CI 0.11, 0.54; p value = 0.002) as compared with phenogroup 3. There was no significant difference in SH events between phenogroups 2 and 3 and no significant interaction between phenogroup membership and randomisation to ILI for the risk of SH events (p-interaction = 0.40).
Association of FMM-based phenogroups with treatment non-adherence
In the derivation cohort, phenogroup 1 participants also had the highest rates of medication non-adherence, with a mean of 2.09 visits during follow-up with <80% medication adherence compared with 1.09 and 1.08 visits for phenogroups 2 and 3, respectively (p value = 0.008) (Fig. 3b). In adjusted analysis, the incidence rate ratio of medication non-adherence was significantly higher in phenogroup 1 compared with phenogroup 3 (0.35; 95% CI 0.10, 0.60) but not compared with phenogroup 2 (0.05; 95% CI –0.12, 0.29; p value = 0.65). Among phenogroup 1 participants randomised to either intensive glycaemic control or combination lipid therapy, medication non-adherence was significantly higher in individuals with a primary composite outcome event on follow-up compared with those without (ESM Table 10).
In the Look AHEAD validation cohort, similar to the derivation cohort, phenogroup 1 had the highest rates of treatment non-adherence with a mean of 26.5% participants with <80% of attended intervention clinic visits compared with 17.9% and 16.4% in phenogroups 2 and 3, respectively (p value < 0.001). Similar to the derivation cohort, among individuals randomised to ILI in phenogroup 1, treatment non-adherence was significantly higher in individuals with a primary composite outcome event compared with those without (ESM Table 10).
Discussion
In this study, several important findings were observed. First, in a cohort of patients with type 2 diabetes enriched for CVD risk, of the four phenomapping approaches tested, the FMM approach was noted to be the most optimal clustering strategy to identify unique phenogroups with distinct cardiometabolic and risk factor profiles. Second, the FMM-based phenogroups had distinct clinical profiles and downstream risk of major CHD events across both the derivation and external validation cohorts. Third, there was a significant interaction between the FMM-based phenogroup and treatment effect of different therapeutic approaches such as intensive glycaemic control, combination lipid therapy, ILI and early coronary revascularisation for the risk of a major CHD event. Finally, we have developed an online, publicly accessible tool for researchers to determine a patient’s phenogroup membership, available at www.cvriskscores.com.
The present study directly compares the performance of multiple clustering strategies for identifying subgroups in a large cohort of patients with type 2 diabetes enriched for CVD risk. A prior study by Ahlqvist et al. identified clusters of individuals with type 2 diabetes that had distinct characteristics and risk of diabetes complications [29]. The clustering technique applied by Ahlqvist et al., and later re-examined by Dennis et al. [30], evaluated phenogroups by focusing on specific pathophysiological abnormalities such as insulin deficiency, ageing and insulin resistance. Similarly, Li et al. used a novel method of topological analysis integrating data from the electronic medical record to identify three subgroups within type 2 diabetes using a proprietary, third-party software platform to perform the analysis [31]. Our study adds to the existing literature by developing and validating a phenomapping approach by leveraging machine-learning-based clustering methods that take into consideration mixed-type data and multiple interactions between covariates. Finally, we were able to compare different clustering methods, including the GMM method that utilised continuous data, LCA that analysed categorical data, and the FMM method and PCA that evaluated mixed data, to identify the most optimal phenomapping approach [32,33,34]. Among these clustering techniques, novel implementation of FMM phenomapping demonstrated the best performance according to BIC, Dunn index and model discrimination in an intermediate CVD risk cohort (ACCORD). Furthermore, FMM phenomapping performed well among participants with type 2 diabetes across different strata of baseline risk ranging from the lower-risk Look AHEAD trial cohort to the higher-risk BARI 2D trial cohort. These findings highlight the generalisability of our phenomapping approach across the spectrum of baseline risk.
Each FMM-based phenogroup had a unique profile of cardiometabolic factors, history of complications and risk of CVD, hypoglycaemic events and medication non-adherence. Phenogroup 1 had the greatest burden of comorbidities, cardiometabolic risk factors and insulin use and the highest risk of adverse events on follow-up. Phenogroup 2 participants had the lowest burden of microvascular complications and had an intermediate risk for CVD and hypoglycaemic events compared with the other subgroups. In contrast, phenogroup 3 participants had a more favourable risk factor profile with the lowest risk of adverse CVD and hypoglycaemic events. The proportion of participants belonging to a specific phenogroup varied across cohorts and was likely related to the baseline CVD risk as well as to the heterogeneity in the population. We further observed that markers of diabetes complications, such as the neuropathy score, filament sensation and urine albumin/creatinine ratio, were the most important covariates for determining phenogroup membership. Our findings add to the existing literature that suggests peripheral neuropathy is associated with an increased risk of adverse cardiovascular events. Similar to our study, Brownrigg et al [35] observed that addition of peripheral neuropathy to existing risk scores significantly improved model discrimination and reclassification. It is possible that the observed benefit in PCE and SNDR model performance with addition of phenogroup membership, as seen in our study, may be due to the addition of new risk factors. Furthermore, our findings support prior studies that identified albuminuria as a key risk factor for CVD among adults with diabetes [36].
We observed substantial heterogeneity in treatment effects of intensive glycaemic control and combination lipid therapy across different phenogroups. Specifically, patients in the lowest-risk phenogroup benefited from intensive glycaemic control and combination lipid therapy in the ACCORD trial dataset. In contrast, participants in the highest-risk group showed potential harm with these interventions. A similar treatment interaction was also observed in the validation cohorts, with greatest benefit of ILI and early coronary revascularisation noted in the low-risk phenogroup 3 of the Look AHEAD and BARI 2D cohorts, respectively. This pattern of lack of benefit from these interventions in the highest-risk group is consistent with prior observations and may be related to presence of a more advanced disease state in which intensive treatment may not modify risk [11, 37, 38]. It is plausible that the trajectory of CVD development is more modifiable in patients with earlier stages of type 2 diabetes with lower burden of associated complications and hypoglycaemic events. This is consistent with prior observations from the United Kingdom Prospective Diabetes Study [39]. Similarly, we also observed that participants in the phenogroup with highest (phenogroup 1) vs lowest (phenogroup 3) risk had higher burden of hypoglycaemic events and the lowest adherence to prescribed medications, which may contribute to the lack of benefit from the tested therapies.
Our study findings have important clinical implications. The phenomapping strategy developed and validated in the present study may help identify distinct subgroups of participants with ‘high-risk’ vs ‘low-risk’ profiles with distinct response to preventive therapies. Such an approach can have important implications for management of patients with type 2 diabetes with regards to resource allocation, downstream follow-up and implementation of targeted preventive strategies. The patients with low-risk profiles may benefit from the therapies tested in the present study including intensive glycaemic control, combination lipid therapy, ILI and early revascularisation. In contrast, the high-risk patients identified by our phenomapping strategy need to be evaluated with newer type 2 diabetes therapies such as SGLT-2 inhibitors, which have been shown to be effective even at the higher end of the baseline CVD risk spectrum [40, 41].
The study has several limitations. First, the phenogroups identified in the present study were derived in an intermediate-risk cohort (ACCORD) that may not be generalisable to most individuals with type 2 diabetes or a more contemporary population on newer therapies such as SGLT-2 inhibitors and GLP-1 receptor agonists. However, we externally validated the phenogroups in low- (Look AHEAD) and high-CVD-risk (BARI 2D) populations and demonstrated consistent patterns of risk and treatment effects. Second, the present study was a post hoc analysis of the ACCORD trial that began enrolment over a decade ago, and prognostic biomarkers, such as natriuretic peptides and high-sensitivity cardiac troponin, and echocardiographic measures of cardiac structure and function were not available for the present analysis [42]. Inclusion of additional phenotypic measures may lead to clustering patterns that differ or improve upon those observed here. Finally, considering the lack of benefit of each of the interventions in the ACCORD, Look AHEAD and BARI 2D trials, the significant treatment interactions observed in the present study should be considered hypothesis generating. Future studies are needed to further validate these observations and test potential targeted interventions in prospective, randomised controlled studies.
In conclusion, among patients with type 2 diabetes, FMM clustering of mixed data was the optimal phenomapping method to identify unique subgroups of patients with distinct cardiovascular risk trajectories and response to established therapeutic approaches. Such semi-supervised clustering techniques may inform development of personalised treatment approaches for patients with type 2 diabetes guided by their baseline risk and unique clinical characteristics.
Data availability
Data from the ACCORD and BARI 2D trials were obtained from the National Institute of Health Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). Look AHEAD trial data were obtained from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Repository.
Abbreviations
- ACCORD:
-
Action to Control Cardiovascular Risk in Diabetes
- aHR:
-
Adjusted HR
- BARI 2D:
-
Bypass Angioplasty Revascularisation Investigation 2 Diabetes
- BIC:
-
Bayesian information criterion
- FMM:
-
Finite mixture model
- GLP-1:
-
Glucagon-like peptide 1
- GMM:
-
Gaussian mixture model
- ILI:
-
Intensive lifestyle intervention
- LCA:
-
Latent class analysis
- Look AHEAD:
-
Look Action for Health in Diabetes
- MI:
-
Myocardial infarction
- MLR:
-
Multinomial logistic regression
- PCA:
-
Principal component analysis
- PCE:
-
Pooled cohort equation
- PPV:
-
Positive predictive value
- SGLT-2:
-
Sodium-glucose cotransporter 2
- SH:
-
Severe/symptomatic hypoglycaemic
- SNDR:
-
Swedish National Diabetes Register
References
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5):1047–1053. https://doi.org/10.2337/diacare.27.5.1047
Gregg EW, Cheng YJ, Srinivasan M et al (2018) Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 391(10138):2430–2440. https://doi.org/10.1016/S0140-6736(18)30314-3
Zelniker TA, Wiviott SD, Raz I et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393(10166):31–39. https://doi.org/10.1016/S0140-6736(18)32590-X
Kristensen SL, Rorth R, Jhund PS et al (2019) Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 7(10):776–785. https://doi.org/10.1016/S2213-8587(19)30249-9
Segar MW, Vaduganathan M, Patel KV et al (2019) Machine learning to predict the risk of incident heart failure hospitalization among patients with diabetes: the WATCH-DM risk score. Diabetes Care 42(12):2298–2306. https://doi.org/10.2337/dc19-0587
Shao H, Fonseca V, Stoecker C, Liu S, Shi L (2018) Novel risk engine for diabetes progression and mortality in USA: Building, relating, assessing, and validating outcomes (BRAVO). Pharmacoeconomics 36(9):1125–1134. https://doi.org/10.1007/s40273-018-0662-1
Berg DD, Wiviott SD, Scirica BM et al (2019) Heart failure risk stratification and efficacy of sodium-glucose Cotransporter-2 inhibitors in patients with type 2 diabetes mellitus. Circulation 140(19):1569–1577. https://doi.org/10.1161/CIRCULATIONAHA.119.042685
Katz DH, Deo RC, Aguilar FG et al (2017) Phenomapping for the identification of hypertensive patients with the myocardial substrate for heart failure with preserved ejection fraction. J Cardiovasc Transl Res 10(3):275–284. https://doi.org/10.1007/s12265-017-9739-z
Guo Q, Lu X, Gao Y et al (2017) Cluster analysis: a new approach for identification of underlying risk factors for coronary artery disease in essential hypertensive patients. Sci Rep 7:43965. https://doi.org/10.1038/srep43965
Shah SJ, Katz DH, Selvaraj S et al (2015) Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 131(3):269–279. https://doi.org/10.1161/CIRCULATIONAHA.114.010637
Segar MW, Patel KV, Ayers C et al (2019) Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur J Heart Fail 22(1):148–158. https://doi.org/10.1002/ejhf.1621
Kao DP, Lewsey JD, Anand IS et al (2015) Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 17(9):925–935. https://doi.org/10.1002/ejhf.327
Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME et al (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358(24):2545–2559. https://doi.org/10.1056/NEJMoa0802743
Bair E, Tibshirani R (2004) Semi-supervised methods to predict patient survival from gene expression data. PLoS Biol 2(4):E108. https://doi.org/10.1371/journal.pbio.0020108
Look Ahead Research Group, Wing RR, Bolin P et al (2013) Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369(2):145–154. https://doi.org/10.1056/NEJMoa1212914
BARI 2D Study Group, Frye RL, August P et al (2009) A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 360(24):2503–2515. https://doi.org/10.1056/NEJMoa0805796
Accord Study Group, Buse JB, Bigger JT et al (2007) Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 99(12A):21i–33i. https://doi.org/10.1016/j.amjcard.2007.03.003
ACCORD Study Group (2016) Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 39(5):701–708. https://doi.org/10.2337/dc15-2283
Ryan DH, Espeland MA, Foster GD et al (2003) Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 24(5):610–628. https://doi.org/10.1016/s0197-2456(03)00064-3
Stekhoven DJ, Buhlmann P (2012) MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics 28(1):112–118. https://doi.org/10.1093/bioinformatics/btr597
Klein JP, Moeschberger ML (2003) Survival analysis: techniques for censored and truncated data, 2nd edn. Springer, New York
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6(2):461–464. https://doi.org/10.1214/aos/1176344136
Dunn JC (1974) Well-separated clusters and optimal fuzzy partitions. J Cybernetics 4(1):95–104. https://doi.org/10.1080/01969727408546059
Goff DC Jr, Lloyd-Jones DM, Bennett G et al (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63(25 Pt B):2935–2959. https://doi.org/10.1016/j.jacc.2013.11.005
Yuan Y, Zhou QM, Li B, Cai H, Chow EJ, Armstrong GT (2018) A threshold-free summary index of prediction accuracy for censored time to event data. Stat Med 37(10):1671–1681. https://doi.org/10.1002/sim.7606
Cederholm J, Eeg-Olofsson K, Eliasson B et al (2008) Risk prediction of cardiovascular disease in type 2 diabetes: a risk equation from the Swedish National Diabetes Register. Diabetes Care 31(10):2038–2043. https://doi.org/10.2337/dc08-0662
Vickers AJ, Elkin EB (2006) Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak 26(6):565–574. https://doi.org/10.1177/0272989X06295361
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20(1):37–46. https://doi.org/10.1177/001316446002000104
Ahlqvist E, Storm P, Karajamaki A et al (2018) Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 6(5):361–369. https://doi.org/10.1016/S2213-8587(18)30051-2
Dennis JM, Shields BM, Henley WE, Jones AG, Hattersley AT (2019) Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol 7(6):442–451. https://doi.org/10.1016/S2213-8587(19)30087-7
Li L, Cheng WY, Glicksberg BS et al (2015) Identification of type 2 diabetes subgroups through topological analysis of patient similarity. Sci Transl Med 7(311):311ra174. https://doi.org/10.1126/scitranslmed.aaa9364
Marbac M, Biernacki C, Vandewalle V (2017) Model-based clustering of Gaussian copulas for mixed data. Communications in Statistics - Theory and Methods 46:11635–11656. https://doi.org/10.1080/03610926.2016.1277753
Marbac M, Sedki M (2017) Variable selection for model-based clustering using the integrated complete-data likelihood. Stat Comput 27(4):1049–1063. https://doi.org/10.1007/s11222-016-9670-1
Marbac M, Sedki M (2019) VarSelLCM: an R/C++ package for variable selection in model-based clustering of mixed-data with missing values. Bioinformatics 35(7):1255–1257. https://doi.org/10.1093/bioinformatics/bty786
Brownrigg JR, de Lusignan S, McGovern A et al (2014) Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart 100(23):1837–1843. https://doi.org/10.1136/heartjnl-2014-305657
Gerstein HC, Mann JF, Yi Q et al (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286(4):421–426. https://doi.org/10.1001/jama.286.4.421
Basu S, Raghavan S, Wexler DJ, Berkowitz SA (2018) Characteristics associated with decreased or increased mortality risk from glycemic therapy among patients with type 2 diabetes and high cardiovascular risk: machine learning analysis of the ACCORD trial. Diabetes Care 41(3):604–612. https://doi.org/10.2337/dc17-2252
Anand IS, Claggett B, Liu J et al (2017) Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail 5(4):241–252. https://doi.org/10.1016/j.jchf.2016.11.015
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359(15):1577–1589. https://doi.org/10.1056/NEJMoa0806470
Fitchett D, Inzucchi SE, Cannon CP et al (2019) Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation 139(11):1384–1395. https://doi.org/10.1161/CIRCULATIONAHA.118.037778
Serenelli M, Bohm M, Inzucchi SE et al (2020) Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur Heart J 41(36):3402–3418. https://doi.org/10.1093/eurheartj/ehaa496
Sharma A, Vaduganathan M, Ferreira JP et al (2020) Clinical and biomarker predictors of expanded heart failure outcomes in patients with type 2 diabetes mellitus after a recent acute coronary syndrome: insights from the EXAMINE trial. J Am Heart Assoc 9(1):e012797. https://doi.org/10.1161/JAHA.119.012797
Acknowledgements
The authors thank the participants, study investigators and staff from the ACCORD, Look AHEAD and BARI 2D trials.
Authors’ relationships and activities
MV serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG and Boehringer Ingelheim; and participates on clinical endpoint committees for studies sponsored by Novartis and the NIH. JB is a consultant for Abbott, Amgen, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa and Vifor. DKM reports honoraria for trial leadership from Astra Zeneca, Sanofi Aventis, Janssen, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk, Lexicon, Eisai, GlaxoSmithKline and Esperion; and honoraria for consulting for Astra Zeneca, Sanofi Aventis, Lilly US, Astra Zeneca, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk and Metavant. AP has served on the advisory board of Roche Diagnostics and has received research support from the Gilead Sciences Research Scholar Program and Applied Therapeutics. The other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
MV is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541). KVP is supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training grant (5T32HL125247-03). DW is supported by the National Center for Advancing Translational Sciences. AP is funded by the Texas Health Resources Clinical Research Scholarship, the Gilead Sciences Research Scholar Program, the National Institute of Aging GEMSSTAR Grant (1R03AG067960-01), and Applied Therapeutics. The study sponsor/funder was not involved in the design of the study; the collection, analysis and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Author information
Authors and Affiliations
Contributions
MWS, KVP and AP conceived and designed the experiment, interpreted the data and wrote the manuscript. MV, MCC, BCJ, MB, DW, JB, PPS, TJW and DKM interpreted the data and revised the manuscript draft for important intellectual content. MWS and AP are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version for publication.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM
(PDF 3.15 mb)
Rights and permissions
About this article
Cite this article
Segar, M.W., Patel, K.V., Vaduganathan, M. et al. Development and validation of optimal phenomapping methods to estimate long-term atherosclerotic cardiovascular disease risk in patients with type 2 diabetes. Diabetologia 64, 1583–1594 (2021). https://doi.org/10.1007/s00125-021-05426-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05426-2