Abstract
Aims/hypothesis
Exposure to sunlight has the potential to suppress metabolic dysfunction and obesity. We previously demonstrated that regular exposure to low-doses of ultraviolet radiation (UVR) reduced weight gain and signs of diabetes in male mice fed a high-fat diet, in part via release of nitric oxide from skin. Here, we explore further mechanistic pathways through which low-dose UVR exerts these beneficial effects.
Methods
We fed mice with a luciferase-tagged Ucp1 gene (which encodes uncoupling protein-1 [UCP-1]), referred to here as the Ucp1 luciferase transgenic mouse (‘Thermomouse’) a high-fat diet and examined the effects of repeated exposure to low-dose UVR on weight gain and development of metabolic dysfunction as well as UCP-1-dependent thermogenesis in interscapular brown adipose tissue (iBAT).
Results
Repeated exposure to low-dose UVR suppressed the development of glucose intolerance and hepatic lipid accumulation via dermal release of nitric oxide while also reducing circulating IL-6 (compared with mice fed a high-fat diet only). Dietary nitrate supplementation did not mimic the effects of low-dose UVR. A single low dose of UVR increased UCP-1 expression (by more than twofold) in iBAT of mice fed a low-fat diet, 24 h after exposure. However, in mice fed a high-fat diet, there was no effect of UVR on UCP-1 expression in iBAT (compared with mock-treated mice) when measured at regular intervals over 12 weeks. More extensive circadian studies did not identify any substantial shifts in UCP-1 expression in mice exposed to low-dose UVR, although skin temperature at the interscapular site was reduced in UVR-exposed mice. The appearance of cells with a white adipocyte phenotype (‘whitening’) in iBAT induced by consuming the high-fat diet was suppressed by exposure to low-dose UVR in a nitric oxide-dependent fashion. Significant shifts in the expression of important core gene regulators of BAT function (Dio2, increased more than twofold), fatty acid transport (increased Fatp2 [also known as Slc27a2]), lipolysis (decreased Atgl [also known as Pnpla2]), lipogenesis (decreased Fasn) and inflammation (decreased Tnf), and proportions of macrophages (increased twofold) were observed in iBAT of mice exposed to low-dose UVR. These effects were independent of nitric oxide released from skin.
Conclusions/interpretation
Our results suggest that non-burning (low-dose) UVR suppresses the BAT ‘whitening’, steatotic and pro-diabetic effects of consuming a high-fat diet through skin release of nitric oxide, with some metabolic and immune pathways in iBAT regulated by UVR independently of nitric oxide.
Similar content being viewed by others

Introduction
Exposure to sunlight or ultraviolet radiation (UVR) has the potential to curb metabolic dysfunction [1]. Repeated exposure to low-dose (non-burning) UVR reduced weight gain and the development of glucose intolerance, insulin resistance, liver steatosis and inflammation in mice fed a high-fat diet [2,3,4]. Only limited benefits of dietary vitamin D supplementation were observed, with effects of UVR independent of changes to serum 25-hydroxyvitamin D (25(OH)D) [3]. Instead, nitric oxide was a mediator partially responsible for some observed metabolic benefits of UVR [2, 3]. Photolabile nitric oxide (e.g. nitrite) is mobilised from skin stores by exposure to UVR, delivered into the circulation as nitrite [3, 5, 6], and linked to hypotensive effects of UVR in humans [7].
Metabolically important deposits of brown adipose tissue (BAT; supraclavicular) lie adjacent to skin often exposed to sunlight (neck, shoulders). BAT contributes to metabolic and temperature homeostasis [8]. BAT dissipates energy as heat (thermogenesis), through uncoupling protein-1 (UCP-1), which uncouples respiration in mitochondria [9]. UCP-1 is highly expressed in BAT and may be induced by a high-fat diet [10]. Ucp1 mRNA levels in BAT exhibit circadian (or daily) rhythms that may be entrained by external cues [11,12,13]. These are linked with the expression of the glucose transporter Glut4 (also known as Slc2a4) and reciprocal changes in molecular clock genes [14]. NEFA [12] and glucose [15] uptake by BAT may also exhibit daily biorhythms. Genetic deletion of Ucp1 shifts circadian rhythms and reduces energy expenditure in mice fed a high-fat diet [16]. These studies suggest that circadian rhythms of UCP-1 expression in BAT may influence thermogenesis and energy expenditure in response to dietary influences.
We hypothesised that regular skin exposure to low-dose UVR activates thermogenic processes in underlying BAT to suppress metabolic dysfunction. A large depot of BAT (interscapular BAT, iBAT) lies directly beneath skin exposed to UVR in our model [2, 3]. To test our hypothesis, we examined the effects of exposure to low-dose UVR in Ucp1 luciferase transgenic (‘Thermomouse’) mice fed a high-fat diet, in which UCP-1 expression in iBAT was tracked, with detailed circadian analyses. We compared the effects of UVR to dietary nitrate, which may promote beiging [17], and skin application of a nitric oxide donor. We used a nitric oxide scavenger to determine whether any effects of UVR were dependent on skin release of nitric oxide.
Methods
See electronic supplementary material (ESM) Methods for more methodological details.
Mice
Experiments were performed according to ethical guidelines of the National Health & Medical and Research Council (Australia) with approval from the Telethon Kids Institute Animal Ethics Committee (AEC numbers 286, 315). Tg(Ucp1-luc2,-tdTomato)1Kajim/J transgenic mice, also known as ‘Thermomouse’, were obtained from the Jackson Laboratory (stock no. 026690; Bar Harbor, ME, USA) and were housed and bred at the Telethon Kids Institute (Subiaco, WA). They are referred to throughout this article as Ucp1 luciferase transgenic mice. Mice were housed under Perspex-filtered fluorescent lighting, with a normal 12 h light/dark cycle (with lights on at 06:00 h) with chipped Aspen bedding, tissues, crinkled paper and PVC piping for nesting in filter-topped cages. These lights do not emit any detectable UVR, as measured using an ultraviolet radiometer (UVX Digital Radiometer, UVP, Upland, CA, USA). Mice had free access to food and water.
Diet
Mice were fed either high-fat (23% lard with canola oil) or low-fat (5% canola oil) diets (ESM Table 1). As previously [3], the lard fraction of the high-fat diet probably contained vitamin D; however, the precise amount is unknown.
UVR
Mice with shaved dorsal skin were irradiated with sub-erythemal/-oedemal ultraviolet B (UVB) radiation (1 kJ/m2; 3.2 ± 0.3 min [mean ± SD]) or mock-irradiated.
Topical skin treatments
Shaved dorsal skin was treated with 0.1 mmol SNAP (S-nitroso-N-acetyl-d,l-penicillamine; nitric oxide donor) alone, or 0.1 mmol cPTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt; nitric oxide scavenger) or vehicle (100 μl) immediately after UVR.
Dietary nitrate supplementation
Nitrate (NaNO3) was administered through drinking water (0.06 mmol kg−1 day−1 NaNO3).
Experiment 1 design
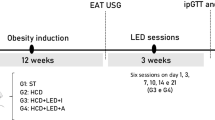
Four-week-old Ucp1 luciferase transgenic male mice were fed the low-fat diet for 4 weeks (Fig. 1). From 8 weeks of age, mice in treatment 1 were fed the low-fat diet, while remaining mice were fed the high-fat diet (treatments 2–6). Mice were treated twice a week with: mock-irradiation then vehicle (treatments 1 and 2); UVR then vehicle (treatment 3); mock-irradiation then SNAP (treatment 4); or UVR then cPTIO (UVR + cPTIO, treatment 5). Mice in treatment 6 were mock-irradiated (with vehicle) and fed nitrate through drinking water. Mice were fed diets and administered treatments for 12 weeks until 20 weeks of age.
Low-dose UVR reduced glucose intolerance through skin release of nitric oxide but did not affect weight gain by Ucp1 luciferase transgenic mice fed the HFD (group-housed). In Experiment 1, 4-week-old Ucp1 luciferase transgenic male mice were fed a low-fat (non-vitamin D-supplemented) diet for 4 weeks. From 8 weeks of age, mice were fed the LFD (treatment 1) or switched to the HFD (treatments 2–6). From 8 weeks of age, the shaved dorsal skin of mice were treated twice a week with: mock-irradiation and then vehicle was applied topically to skin (treatments 1 and 2); sub-erythemal UVR (1 kJ/m2 UVB) and then vehicle (treatment 3); mock-irradiation and then the nitric oxide donor SNAP (0.1 mmol) was applied topically to skin (SNAP, treatment 4); or, sub-erythemal UVR and then the nitric oxide scavenger cPTIO (0.1 mmol) was applied topically to skin (UVR + cPTIO, treatment 5). Mice that received treatment 6 were mock-irradiated and fed nitrate (0.06 mmol kg−1 day−1) through their drinking water. Mice were fed the LFD or HFD and administered the treatments twice a week for 12 weeks until 20 weeks of age. (a) Overview of this experiment. (b, c) Mice were weighed weekly. Body weight (n ≥ 21) (b) and weight gain (n ≥ 21) (c) post intervention. (d, e) A GTT was conducted at 10 weeks post intervention, with blood glucose levels (n ≥ 7) (d) and AUCs (e) determined. Data are shown as mean ± SEM; *p < 0.05 (one-way ANOVA with Tukey’s post hoc analysis) for mice fed the LFD vs all other treatments (b–d) and between the indicated groups (e). HFD, high-fat diet; LFD, low-fat diet
Experiment 2 design
Four-week-old mice were individually housed and fed a low-fat diet for 4 weeks (Fig. 5a). From 8 weeks of age, mice in treatment 1 were fed the low-fat diet, while remaining mice were fed the high-fat diet (treatments 2–4). Mice were treated twice a week with: mock-irradiation (treatments 1 and 2) with vehicle; UVR then vehicle (treatment 3); or UVR then cPTIO (UVR + cPTIO, treatment 4). Mice were fed diets and administered treatments for 12 weeks until 20 weeks of age.
Measuring weight gain, tissue weights and food intake
Weekly percentage weight gain was calculated from body weight at 8 weeks of age, with liver, gonadal white adipose tissue (WAT) and iBAT weights determined at the end of experiments. Food and energy intake was determined in Experiment 1, by weighing diet present in food hoppers.
Glucose and insulin tolerance tests
Fasted mice were intraperitoneally challenged with glucose or insulin for glucose or insulin tolerance tests (GTT/ITT). Glucose was measured before injection and 15, 30, 45, 60, 90 and 120 min post injection.
Serum lipids and metabolites
Serum (total, HDL-, and LDL-) cholesterol, triacylglycerol, calcium, 25(OH)D, IL-6 and TNF-α, and plasma insulin and adiponectin (both from fasting mice) were quantified as detailed in ESM Methods. Serum nitrite and nitrate levels were measured as previously described [18].
Histopathological assessment of liver and iBAT
Liver sections were stained with either H&E, Masson’s trichrome or Oil Red O with signs of non-alcoholic fatty liver disease (NAFLD) and lipid content quantified in a blinded fashion. Sections of iBAT were stained with H&E and blindly scored for an increasing white adipocyte phenotype (‘whitening’).
UCP-1 expression
In a preliminary experiment, real-time measurement of UCP-1 expression (bioluminescence, measured post luciferin injection) was quantified in iBAT at 0, 0.25 (15 min), 3, 24, 48 and 192 h after 8-week-old mice fed a low-fat diet were exposed to 1 kJ/m2 UVR. In Experiment 1 (Fig. 1a), UCP-1 expression was quantified in iBAT at baseline (8 weeks of age), prior to diet and skin treatments, and at 1, 4, 8 and 12 weeks post intervention. In Experiment 2 (Fig. 5a), daily UCP-1 expression (7 times in 28 h) was quantified in iBAT at baseline (8 weeks of age), prior to beginning (and 24 h following) diet and skin treatments, and at 6 and 12 weeks post intervention.
Interscapular skin temperature
In Experiment 2, an infrared skin thermometer was used to measure the temperature at the shaved interscapular skin site above the iBAT deposit.
Blood glucose
In Experiment 2, immediately prior to each luciferin injection, glucose concentrations were determined in a drop of blood from the tail vein.
Mitochondria in iBAT
At the end of Experiment 1, mitochondrial content (amount) and activity (membrane potential) was quantified using confocal microscopy.
Immune cells in vascular stromal compartment of iBAT
At the end of Experiment 1, macrophages and regulatory T cells were phenotyped using flow cytometry.
Detection of mRNA
At the end of Experiment 2, mRNA levels were quantified in iBAT using real-time PCR for the following gene targets: Eef1α (also known as Eef1a1-ps1) (housekeeping), Atgl, Bmp7, Cd36, Cidea, Dio2, Ebf2, Fasn, Fatp2, Fgf21, Glut4, Il6, Pgc-1α (also known as Ppargc1a), Pla2 (also known as Pla2g2a), Pparγ (also known as Pparg), Prdm16, Tnf, Ucp1 and Zic1.
Statistical analyses
p values < 0.05 were considered statistically significant. Generally, unless otherwise stated, data were compared using ANOVA with Tukey’s post hoc (if normally distributed) or Kruskal–Wallis test with Dunn’s post hoc (if not normally distributed) analyses to define differences between treatments.
Results
Chronic exposure to low-dose UVR did not modify weight gain or WAT weight in mice fed high-fat diet
In the group-housed mice in Experiment 1, Ucp1 luciferase transgenic male mice fed a high-fat diet weighed more (by mean 2.4 g, p = 0.02) and had greater weight gain (by 7.5%, p = 0.004) after 10 weeks compared with mice fed a low-fat diet (Fig. 1b, c, Table 1). There was no effect of repeated exposure to UVR, or any other treatments, on body weight or weight gain or gonadal WAT weight compared with mice fed the high-fat diet only.
Repeated exposure to UVR suppressed glucose intolerance
After 10 weeks, fasting glucose levels were elevated by 43% in mice fed a high-fat diet compared with the low-fat diet (p = 0.01), with no effect of UVR (Table 2). Increased glucose intolerance observed in mice fed the high-fat diet (p = 0.002) was suppressed by UVR (p = 0.04, Fig. 1d, e). These effects of UVR were reversed by topical cPTIO, a nitric oxide scavenger (p = 0.03, Fig. 1d, e). Topical SNAP (nitric oxide donor) did not affect glucose levels in mice fed the high-fat diet (p = 0.99, Fig. 1d, e). Thus, low-dose UVR suppressed glucose intolerance through skin release of nitric oxide.
UVR effects on insulin resistance in mice fed high-fat diet
Increased insulin resistance (AUC; week 11) was observed in mice fed the high-fat diet compared with low-fat diet (p = 0.001, Table 2). UVR reduced, but not significantly, the degree of insulin resistance observed in mice fed the high-fat diet (p = 0.06). Fasting insulin or adiponectin levels (week 9) were increased by the high-fat diet (compared with low-fat diet, Mann–Whitney U test, p ≤ 0.01), but were not affected by UVR or SNAP (Table 2).
UVR did not alter serum lipid levels or food intake
At the end of Experiment 1, total- and HDL-cholesterol levels were increased in mice fed a high-fat diet compared with low-fat diet (by ~20%, p ≤ 0.0001). There was no effect of UVR or SNAP on serum lipids compared with mice fed the high-fat diet only (p > 0.16, Table 3). Food/energy intake was not significantly modified by exposure to UVR in mice fed the high-fat diet (p > 0.1, ESM Table 2).
UVR suppressed serum IL-6 levels and improved vascular nitric oxide
IL-6 (but not TNF-α) measured at week 12 was increased in mice fed the high-fat diet compared with low-fat diet (by 69%, p = 0.03, Student’s t test) and reduced by UVR or SNAP (by 58%, p = 0.02, Table 3). The effects of UVR were not reversed by cPTIO. Serum 25(OH)D levels were increased in mice fed the high-fat diet (ESM Table 3), as previously observed [3]. UVR did not increase serum 25(OH)D as previously observed and extensively characterised in male mice fed low- or high-fat diets supplemented (or not) with vitamin D [3, 19]. Similarly, in previous studies, 1,25-dihydroxyvitamin D levels were not increased in irradiated skin of male mice exposed to UVR, although a lack of response could have been due to the limits of detection of the immunoassay used [19]. A small increase in serum calcium occurred in UVR-exposed mice (by 4%, p = 0.04). Circulating nitrite concentrations were reduced in mice fed a high-fat diet compared with low-fat diet (by 38%, p < 0.0001, Student’s t test), indicative of impaired vascular nitric oxide production and endothelial dysfunction [20]. UVR, SNAP and dietary nitrate all tended to normalise nitrite concentrations (only significantly for SNAP, p = 0.04, ESM Table 3).
Low-dose UVR suppressed signs of NAFLD in mice fed high-fat diet
Livers of mice fed a high-fat diet had increased lipid content (by 95%, p = 0.02, Fig. 2b, m), which was reduced by UVR (by 80%, p = 0.02, Fig. 2c, m); an effect reversed by cPTIO (p = 0.02, Fig. 2h, m). SNAP had suppressive effects on liver lipid content similar to UVR (although not significant, p = 0.07, Fig. 2g, m). Similar findings were obtained when the extent of steatosis, hepatocellular ballooning and fibrosis were compared (Fig. 2d–f, j–l, n).
Low-dose UVR prevented liver steatosis through a nitric oxide-dependent mechanism (in group-housed mice). In Experiment 1, 4-week-old male Ucp1 luciferase transgenic mice were fed the LFD for 4 weeks. At 8 weeks of age mice were separated into one of six treatment groups: (1) LFD; (2) HFD; (3) HFD with UVR (UVR); (4) HFD with topical SNAP (SNAP); (5) HFD with UVR followed by topical cPTIO (UVR + cPTIO); or (6) HFD with nitrate in drinking water (Nitrate) (see Fig. 1 for details). After 12 weeks of treatment, livers were histopathologically assessed. Representative sections (×20 oil immersion objective; scale bar, 10 μm) are shown for Oil Red O-stained (a–c and g–i) or Masson’s trichrome-stained (d–f and j–l) liver samples obtained from mice from the: (a, d) LFD; (b, e) HFD; (c, f) UVR; (g, j) SNAP; (h, k) UVR + cPTIO; and (i, l) Nitrate treatments. In (m), lipid content was scored following the staining of liver sections with Oil Red O (HFD vs SNAP; p = 0.07). In (n), livers were histopathologically assessed following staining with H&E or Masson’s trichrome with a combined score for steatosis, hepatocellular ballooning and fibrosis shown. Data in (m) and (n) are shown as mean ± SEM (n ≥ 5/treatment); *p < 0.05 (one-way ANOVA with Tukey’s post hoc analysis). HFD, high-fat diet; LFD, low-fat diet
Dietary nitrate did not reproduce suppressive effects of low-dose UVR
Dietary nitrate did not reproduce any of the suppressive effects of UVR (or SNAP) on metabolic outcomes described in Figs 1, 2, and Tables 1, 2, 3, with liver weights increased compared with mice fed a high-fat diet (p = 0.01, Table 1). Serum levels of nitrate (not nitrite) were substantially increased by dietary nitrate (~six–ninefold, p < 0.0001, ESM Table 3). Together, these findings suggest that skin release of nitric oxide (induced by UVR, or chemically) may be a more potent way of regulating metabolic dysfunction than dietary nitrate.
UVR did not modify UCP-1 expression in iBAT in mice fed high-fat diet
In a preliminary experiment, UCP-1 expression peaked in iBAT 24 h after exposure of adult mice (fed the low-fat diet) to a single 1 kJ/m2 dose of UVR (by more than twofold, p = 0.03, Fig. 3). At week 12 in Experiment 1 (Fig. 1a), there was some evidence for increased UCP-1 expression in iBAT of mice fed a high-fat diet, compared with low-fat diet (Table 4; p = 0.07, Student’s t test). In mice fed the high-fat diet, exposure to low-dose UVR did not modulate UCP-1 expression in iBAT (Table 4). Interestingly, UCP-1 expression was reduced in the iBAT of mice topically treated with cPTIO at week 12 (Table 4). Treatment with dietary nitrate transiently increased UCP-1 levels at the subcutaneous WAT site (lower back, inguinal), with increased levels observed at week 8 only (low-fat diet = 2.9 ± 0.3; high-fat diet = 3.2 ± 0.4; UVR = 3.3 ± 0.3; SNAP = 2.4 ± 0.1; UVR + cPTIO = 3.8 ± 0.7; nitrate = 8.8 ± 0.2; mean ± SEM × 103 peak radiance, p < 0.05), but not other times (data not shown). We observed no effect of feeding mice a high-fat diet or UVR on mitochondrial content or membrane potential in iBAT at week 12 (ESM Fig. 1).
UCP-1 expression in iBAT peaked 24 h after exposure of Ucp1 luciferase transgenic mice to low-dose UVR. Four-week-old male Ucp1 luciferase transgenic mice were fed an LFD. At 8 weeks of age, mice were exposed to a single dose of UVR (1 kJ/m2) with UCP-1 levels in iBAT (red circles) tracked over 192 h. (a) Representative mice are shown at baseline, and 0.25 (15 min), 3, 24, 48 and 192 h post exposure, with (b) the peak radiance levels of bioluminescence at the iBAT site. (b) Data are shown as mean ± SEM (n = 8/treatment); *p < 0.05 compared with baseline (one-way ANOVA with Tukey’s post hoc analysis). LFD, low-fat diet
UVR reduced ‘whitening’ of iBAT induced by high-fat diet
At week 12, iBAT obtained from mice fed a high-fat diet weighed more (Table 1) and exhibited increased ‘whitening’ (white adipocyte phenotype of BAT [21], Fig. 4b, g) compared with mice fed a low-fat diet (p = 0.006, Fig. 4a, g). UVR reduced iBAT weights (p = 0.02, Table 1) and degree of whitening of iBAT (p < 0.0001, Fig. 4c, g) in mice fed the high-fat diet. Effects of UVR were reversed by cPTIO (Table 1; Fig. 4e, g), but not reproduced by SNAP (Fig. 4d) or dietary nitrate (Fig. 4f). There was a moderate and significant linear correlation between the hepatic lipid content and iBAT whitening (Spearman’s correlation, r = 0.428, p = 0.002). These data suggest that exposure to low-dose UVR prevented fat accumulation in the liver and the whitening of iBAT, through skin release of nitric oxide. There was also an increase (by more than twofold) in proportions of macrophages (F4/80+MHC class II+ cells [22]) in iBAT of mice exposed to UVR or SNAP, compared with mice fed the high-fat diet only (ESM Fig. 2). More substantial increases in F4/80+MHC class II+ cells were observed in mice supplemented with nitrate, with a (non-significantly) reduced percentage expressing CD86, and less CD11c and CD301 (macrophage type-1 and -2 markers [23]) per cell (ESM Fig. 2). There were no differences between treatments in proportions of regulatory CD4+ T cells expressing FOXP3, CD25 and/or IL-10 in iBAT (data not shown).
The extent of whitening of iBAT induced by feeding mice an HFD was suppressed by exposure to low-dose UVR. In Experiment 1, 4-week-old male Ucp1 luciferase transgenic mice were fed an LFD for 4 weeks. At 8 weeks of age mice were separated into one of six treatment groups: (1) LFD; (2) HFD); (3) HFD with UVR (UVR); (4) HFD with topical SNAP (SNAP); (5) HFD with UVR followed by topical cPTIO (UVR + cPTIO); or (6) HFD with nitrate in drinking water (Nitrate) (see Fig. 1 for details). After 12 weeks of treatment, iBAT was histopathologically assessed for the extent of whitening following staining with H&E, with representative iBAT tissue sections shown for mice from the (a) LFD, (b) HFD, (c) UVR, (d) SNAP, (e) UVR + cPTIO, and (f) Nitrate treatments (×20 oil immersion objective; scale bar, 10 μm), and with histology scores for the extent of whitening (WAT phenotype score) (g), in which data are shown as mean ± SEM (n ≥ 5/treatment); *p < 0.05 (one-way ANOVA with Tukey’s post hoc analysis). In (b) the blue arrows point to cells with a white adipocyte phenotype. HFD, high-fat diet; LFD, low-fat diet
Determining the effects of low-dose UVR on biorhythms of UCP-1
In Experiment 1, we measured UCP-1 expression at a single time of day (between 10:00 and 14:00) at each timepoint. Thus, our inability to detect robust changes in UCP-1 expression in response to dietary (high-fat) or skin (UVR) treatment (Table 4) may have been because we did not consider circadian rhythm. In Experiment 2, we examined the daily biorhythms of UCP-1 expression in iBAT prior to, and after, 6 and 12 weeks of treatments, with data collected across 28 h at each timepoint and mice exposed to UVR (or high-fat diet at baseline) at Zeitgeber time (ZT)3 (09:00; see ‘UCP-1 expression’ in ESM Methods for further information on how ZTs relate to clock times) (Fig. 5a).
Low-dose UVR reduced NAFLD pathophysiology in Ucp1 luciferase transgenic mice fed an HFD (individually housed, with data collected for circadian rhythm analysis). In Experiment 2, 4-week-old Ucp1 luciferase transgenic male mice were fed a low-fat (non-vitamin D-supplemented) diet for 4 weeks. From 8 weeks of age, mice were fed the LFD (treatment 1) or switched to an HFD (treatments 2–4). From 8 weeks of age, the shaved dorsal skin of mice was treated twice a week with: mock-irradiation and then vehicle applied to skin (treatments 1 and 2); sub-erythemal UVR (1 kJ/m2 UVB) and then vehicle (treatment 3); or sub-erythemal UVR and then the nitric oxide scavenger cPTIO (0.1 mmol) was applied to skin (UVR + cPTIO, treatment 4). Mice were fed an LFD or HFD and administered the treatments twice a week for 12 weeks until 20 weeks of age. At baseline (prior to feeding mice the HFD or skin treatments), and after 6 and 12 weeks of treatment, data were collected for circadian analyses of UCP-1 bioluminescence in iBAT, interscapular skin temperature and blood glucose (as indicated at the clocks below the timeline in a, and at time 0 and as indicated by red dashed lines in b and c). Data were collected across 28 h at each timepoint and mice exposed to UVR (or high-fat diet at baseline) at Zeitgeber time (ZT)3 (09:00). (a) Overview of the experiment. (b, c) Mice were weighed weekly. Body weight (n = 20) (b) and weight gain (n = 20) (c) post intervention. (d) After 12 weeks of treatment, livers were histopathologically assessed following staining with H&E or Masson’s trichrome with a combined score for steatosis, hepatocellular ballooning and fibrosis shown as mean ± SEM (n = 10/treatment). A GTT was conducted at 10 weeks post intervention with (e) AUCs of the GTT (n = 8/treatment) determined. Data are shown as mean ± SEM; *p < 0.05 (one-way ANOVA with Tukey’s post hoc analysis) for mice fed an LFD vs all other treatments (b–c); and between indicated groups (d). HFD, high-fat diet; LFD, low-fat diet
High-fat diet increased adiposity and signs of NAFLD in mice housed individually
Increased body weight (p = 0.001, Fig. 5b) and weight gain (p = 0.001, Fig. 5c) were observed after 1 week of feeding mice the high-fat diet, which also had greater WAT depot weights at week 12 (Table 5). There were some transient reductions in body weight and weight gain in response to each circadian rhythm analysis (Fig. 5b, c). Worse liver histology score was observed in mice fed the high-fat diet (Fig. 5d). However, there was limited evidence for metabolic dysfunction when mice were housed individually with the high-fat diet not increasing fasting insulin or glucose, or glucose levels when measured during the GTT or ITT (Table 6, Fig. 5e). However, in the circadian analyses, (non-fasting) blood glucose levels were increased in mice fed a high-fat diet (Fig. 6e: from ZT5, week 6).
Detailed circadian expression analyses of UCP-1 in iBAT, interscapular skin temperature and blood glucose (in individually housed mice). In Experiment 2, 4-week-old male Ucp1 luciferase transgenic mice were fed an LFD for 4 weeks. From 8 weeks of age mice were separated into one of four treatment groups: (1) LFD; (2) HFD; (3) HFD with UVR (UVR); or (4) HFD with UVR followed by topical cPTIO (UVR + cPTIO). At baseline (prior to feeding mice the HFD or skin treatments) (a–c), and after 6 (d–f) and 12 (g–i) weeks of treatment, data were collected for circadian analyses of UCP-1 bioluminescence in iBAT (a, d, g), blood glucose (b, e, h) and interscapular skin temperature (c, f, i) at intervals of 4–6 h over 28 h. A grey dashed line depicts when the dietary and skin interventions began at baseline (a–c, HFD UVR box), and when a skin treatment (UVR box) was performed at weeks 6 (d–f) and 12 (g–i). Data are shown as mean ± SEM (n = 12 for UCP-1 bioluminescence, and n = 20 for temperature and blood glucose per treatment); *p < 0.05 for HFD vs LFD; †p < 0.05 for HFD vs UVR; ‡p < 0.05 for UVR vs cPTIO (two-way ANOVA with Tukey’s post hoc analysis). HFD, high-fat diet; LFD, low-fat diet
Low-dose UVR suppressed gonadal WAT and iBAT weights and signs of NAFLD in mice housed individually
While UVR did not affect body weight (Fig. 5b) or weight gain (Fig. 5c), gonadal WAT and iBAT weights (Table 5) and liver histology score (Fig. 5d) were reduced in mice exposed to UVR, compared with mice only fed the high-fat diet. These effects of UVR on liver histopathology (Fig. 5d), but not WAT or iBAT weights (Table 5), were reversed by cPTIO, pointing towards nitric oxide-dependent and -independent effects.
UCP-1 expression in iBAT increased by high-fat diet in mice housed individually
There were minimal effects of eating a high-fat diet on iBAT UCP-1 expression at baseline (Fig. 6a) and 6 weeks (Fig. 6d). By 12 weeks, UCP-1 levels were increased in iBAT of mice fed a high-fat diet at nearly all times (except ZT9, 15:00, Fig. 6g, *p < 0.05). Similarly, there were more distinct increases in blood glucose levels after 12 weeks (Fig. 6h, *p < 0.05) of high-fat diet feeding, than at baseline (Fig. 6b), or 6 weeks (Fig. 6e). While no significant cosinor rhythms in the expression of UCP-1 in iBAT or glucose levels in blood were detected (ESM Table 4), levels varied across the day; e.g. in mice fed a high-fat diet for 12 weeks, UCP-1 was increased at ZT3 (24 h post UVR), compared with ZT9 and ZT13 (Fig. 6g, one-way ANOVA, p < 0.05).
Low-dose UVR had minimal effects on UCP-1 expression in iBAT and blood glucose in mice housed individually
There was no effect of low-dose UVR on the expression of UCP-1 in iBAT or blood glucose levels in mice fed a high-fat diet at any time, except at week 12 at ZT3 (24 h post UVR), when UCP-1 levels were reduced (Fig. 6g, p < 0.05). This effect was not reversed by cPTIO (Fig. 6g). These findings suggest that the capacity for UVR to reduce gonadal WAT (Table 5) and prevent liver pathophysiology (Fig. 5d) were unlikely to occur through changes in UCP-1-mediated thermogenesis in iBAT, or pathways that regulate blood glucose.
Low-dose UVR reduced interscapular skin temperature in mice fed high-fat diet
After 6 (Fig. 6f) and 12 (Fig. 6i) weeks (but not at baseline, Fig. 6c), interscapular skin temperatures were reduced in mice fed a low-fat diet (week 6 at ZT13, ZT3 [24 h post UVR]; week 12, ZT23; p < 0.05), and mice exposed to low-dose UVR (week 6 at ZT13, p < 0.05), compared with mice fed a high-fat diet (only). Interscapular skin temperatures were reduced in mice exposed to UVR, compared with those treated with cPTIO (Fig. 6f, week 6 at ZT13; Fig. 6i, week 12, ZT1, ZT9, ZT23; p < 0.05). At 12 weeks, there were no differences in interscapular skin temperature measured between mice fed the low-fat diet, and those exposed to UVR (Fig. 6i; p > 0.05). There were no significant linear correlations (p > 0.05) between UCP-1 expression in iBAT, and interscapular skin temperature in mice of any treatment. These data suggest repeated exposure of mice fed a high-fat diet to low-dose UVR reduced interscapular skin temperatures (at the irradiated site), through mechanisms dependent on skin release of nitric oxide.
UVR increased Dio2, Glut4 and Fatp2 and reduced Atgl, Fasn and Tnf mRNAs in iBAT
At the end of Experiment 2, exposure to low-dose UVR ‘normalised’ mRNA levels of some core gene regulators of BAT function, namely Pgc1α, Pparγ and Ucp1, with levels similar to those observed in mice fed the low-fat diet (ESM Fig. 3). Levels of other core gene regulators, Prdm16, Ebf2, Bmp7 and Zic1, were reduced by the high-fat diet and not modulated by UVR, with similar findings for genes regulating fatty acid storage (Cidea) and transport (Cd36), and a batokine (Fgf21). However, mRNAs of a core gene regulator (Dio2, deiodinase, iodothyronine, type II) and glucose and fatty acid transporters (Glut4, solute carrier family 2 [facilitated glucose transporter], member 4; Fatp2, solute carrier family 2 [facilitated glucose transporter], member 2) were increased in iBAT of mice exposed to UVR. Levels of Fasn (fatty acid synthase, a lipogenesis gene), Atgl (adipose triglyceride lipase, a lipolysis gene) and Tnf mRNAs were reduced in mice exposed to UVR, compared with those only fed the high-fat diet. These observations were independent of nitric oxide release from UV-irradiated skin.
Discussion
Here we provide evidence for metabolic benefits of low-dose UVR, which promoted glucose tolerance, and reduced hepatic fat accumulation, whitening of iBAT, and blood IL-6 levels. Some effects of low-dose UVR were different in mice housed individually (e.g. reduced gonadal WAT weights compared with co-housed mice). Dermal release of nitric oxide was responsible for the protective effects of UVR in reducing glucose intolerance and hepatic fat accumulation (and whitening of iBAT for co-housed mice). Exposure to UVR enhanced expression of Dio2 in iBAT of mice fed the high-fat diet. This gene can be activated via bile acids produced by the liver, and promotes expression of peroxisome proliferator-activated receptor-γ cofactor-1α (PGC1α) and mitochondrial biogenesis in BAT (reviewed by [24]). These findings suggest that there may be novel mechanistic links between the liver and BAT regulated by UVR. While increased UCP-1 levels in iBAT were observed 24 h after exposure to low-dose UVR in mice fed a low-fat diet, low-dose UVR did not raise UCP-1 levels in iBAT at any time in our detailed circadian analyses in mice fed a high-fat diet. Instead, chronic exposure to low-dose UVR reduced interscapular skin temperatures, an effect that was reversed by topical cPTIO, suggesting a role for UVR-induced nitric oxide.
Dermal interscapular temperatures may only partially reflect thermogenesis in iBAT [25, 26], with further contributions by local vasomotor tone [26], which can influence thermoregulation in rodents [27]. Dermal vasomotor activity depends partially on core body temperature [26] as suggested in our study with skin temperatures >36°C. However, UVR increased forearm blood flow in healthy young men, independently of dermal skin temperature [28]. Both diet and sun exposure may alter vascularisation of the dermis, which is increased by caloric restriction [29], while reduced lymphatic vessel density occurs in sun-damaged skin [30]. Although we did not observe any signs of increased UCP-1-mediated thermogenesis with UVR, there was reduced whitening of iBAT, and lower Tnf mRNA levels, but more macrophages. Whitening of BAT may induce crown-like structures and macrophage infiltration [31]. Other unexpected findings were that mRNAs of lipolysis and lipogenesis genes, Atgl and Fasn, respectively, were reduced, while Glut4 and Fatp2 mRNAs increased with UVR. More studies are needed to further characterise the effects of UVR on these pathways in iBAT, and to establish if there are links to the anti-inflammatory effects of UVR.
As we demonstrate here, to gain better biological insights into processes that govern thermogenesis, some consideration of variability induced by time of day is needed. Other important considerations include: non-thermoneutral conditions [10]; cage density; experimental stress; sex (the Ucp1 luciferase transgene is inserted into the Y chromosome [9]); and genetic background (e.g. FVB/NJ mice have restricted weight gain [32]). Our findings in Ucp1 luciferase transgenic mice (FVB/NJ background) are mostly reminiscent of previous observations in C57Bl/6J mice [3], suggesting that the effects of low-dose UVR are not limited to a single mouse strain, and may be effective in genetically diverse humans. While proton (H+) leak in mitochondria may be mediated by proteins other than UCP-1 [33], this process is thought to be the main driver of heat production by iBAT [34]. Additional mechanisms may include proton leak and/or OH- channel activation through allosteric binding of long-chain fatty acids to UCP-1 [34], and UCP-1-independent pathways, such as creatinine cycling, the glycerol-3-phosphate shuttle and the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pathway [35].
In conclusion, we report beneficial effects of low-dose UVR on metabolic outcomes in Ucp1 luciferase transgenic mice fed a high-fat diet. The suppressive effects of UVR on glucose intolerance, hepatic fat accumulation, whitening of iBAT and interscapular skin temperatures were dependent on release of nitric oxide from irradiated skin. Detailed circadian analyses did not reveal substantial differences in UCP-1 expression in iBAT, suggesting that beneficial metabolic effects of UVR are mediated via another route. We also observed that dermal release of nitric oxide may be a more effective means of reducing metabolic dysfunction than dietary manipulation. Further studies examining the effects of exposure to UVR on glucose and lipid metabolic pathways in iBAT and other tissues, and its effects on vascular tone and temperature of the dermis, are needed.
Data availability
Data are available on request to authors.
Abbreviations
- BAT:
-
Brown adipose tissue
- cPTIO:
-
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt
- 25(OH)D:
-
25-Hydroxyvitamin D
- iBAT:
-
Interscapular BAT
- NAFLD:
-
Non-alcoholic fatty liver disease
- SNAP:
-
S-nitroso-N-acetyl-d,l-penicillamine
- UVB:
-
Ultraviolet B
- UVR:
-
Ultraviolet radiation
- WAT:
-
White adipose tissue
- ZT:
-
Zeitgeber time
References
Gorman S, Lucas RM, Allen-Hall A, Fleury N, Feelisch M (2017) Ultraviolet radiation, vitamin D and the development of obesity, metabolic syndrome and type-2 diabetes. Photochem Photobiol Sci 16(3):362–373. https://doi.org/10.1039/c6pp00274a
Fleury N, Feelisch M, Hart PH et al (2017) Sub-erythemal ultraviolet radiation reduces metabolic dysfunction in already overweight mice. J Endocrinol 233(1):81–92. https://doi.org/10.1530/JoE-16-0616
Geldenhuys S, Hart PH, Endersby R et al (2014) Ultraviolet radiation suppresses obesity and symptoms of metabolic syndrome independently of vitamin D in mice fed a high-fat diet. Diabetes 63(11):3759–3769. https://doi.org/10.2337/db13-1675
Teng S, Chakravorty L, Fleury N, Gorman S (2019) Regular exposure to non-burning ultraviolet radiation reduces signs of non-alcoholic fatty liver disease in mature adult mice fed a high fat diet: results of a pilot study. BMC Res Notes 12(1):78–78. https://doi.org/10.1186/s13104-019-4112-8
Oplander C, Suschek CV (2012) The role of photolabile dermal nitric oxide derivates in ultraviolet radiation (UVR)-induced cell death. Int J Mol Sci 14(1):191–204. https://doi.org/10.3390/ijms14010191
Mowbray M, McLintock S, Weerakoon R et al (2009) Enzyme-independent NO stores in human skin: quantification and influence of UV radiation. J Invest Dermatol 129(4):834–842. https://doi.org/10.1038/jid.2008.296
Liu D, Fernandez BO, Hamilton A et al (2014) UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. J Invest Dermatol 134(7):1839–1846. https://doi.org/10.1038/jid.2014.27
Stanford KI, Middelbeek RJ, Townsend KL et al (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123(1):215–223. https://doi.org/10.1172/JCI62308
Galmozzi A, Sonne SB, Altshuler-Keylin S et al (2014) ThermoMouse: an in vivo model to identify modulators of UCP1 expression in brown adipose tissue. Cell Rep 9(5):1584–1593. https://doi.org/10.1016/j.celrep.2014.10.066
Feldmann HM, Golozoubova V, Cannon B, Nedergaard J (2009) UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9(2):203–209. https://doi.org/10.1016/j.cmet.2008.12.014
Razzoli M, Emmett MJ, Lazar MA, Bartolomucci A (2018) Beta-adrenergic receptors control brown adipose UCP-1 tone and cold response without affecting its circadian rhythmicity. FASEB J 32(10):5640–5646. https://doi.org/10.1096/fj.201800452R
van den Berg R, Kooijman S, Noordam R et al (2018) A diurnal rhythm in brown adipose tissue causes rapid clearance and combustion of plasma lipids at wakening. Cell Rep 22(13):3521–3533. https://doi.org/10.1016/j.celrep.2018.03.004
Eley J, Himms-Hagen J (1989) Brown adipose tissue of mice with GTG-induced obesity: altered circadian control. Am J Physiol 256(6 Pt 1):E773–E779. https://doi.org/10.1152/ajpendo.1989.256.6.E773
Lee P, Bova R, Schofield L et al (2016) Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab 23(4):602–609. https://doi.org/10.1016/j.cmet.2016.02.007
van der Veen DR, Shao J, Chapman S, Leevy WM, Duffield GE (2012) A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET-FDG imaging. Obesity (Silver Spring) 20(7):1527–1529. https://doi.org/10.1038/oby.2012.78
Luijten IHN, Feldmann HM, von Essen G, Cannon B, Nedergaard J (2019) In the absence of UCP1-mediated diet-induced thermogenesis, obesity is augmented even in the obesity-resistant 129S mouse strain. Am J Physiol Endocrinol Metab 316(5):E729–E740. https://doi.org/10.1152/ajpendo.00020.2019
Roberts LD, Ashmore T, Kotwica AO et al (2015) Inorganic nitrate promotes the browning of white adipose tissue through the nitrate-nitrite-nitric oxide pathway. Diabetes 64:471–484. https://doi.org/10.2337/db14-0496
Milsom AB, Fernandez BO, Garcia-Saura MF, Rodriguez J, Feelisch M (2012) Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of NO signaling products. Antioxid Redox Signal 17(3):422–432. https://doi.org/10.1089/ars.2011.4156
Gorman S, Scott NM, Tan DH et al (2012) Acute erythemal ultraviolet radiation causes systemic immunosuppression in the absence of increased 25-hydroxyvitamin D3 levels in male mice. PLoS One 7(9):e46006. https://doi.org/10.1371/journal.pone.0046006
Lauer T, Preik M, Rassaf T et al (2001) Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A 98(22):12814–12819. https://doi.org/10.1073/pnas.221381098
Gao M, Ma Y, Liu D (2015) High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One 10(3):e0119784. https://doi.org/10.1371/journal.pone.0119784
Farrell HE, Bruce K, Lawler C, Stevenson PG (2019) Murine cytomegalovirus spread depends on the infected myeloid cell type. J Virol 93(15): pii: e00540-19. https://doi.org/10.1128/JVI.00540-19
Braune J, Weyer U, Hobusch C et al (2017) IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J Immunol 198(7):2927–2934. https://doi.org/10.4049/jimmunol.1600476
Braun K, Oeckl J, Westermeier J, Li Y, Klingenspor M (2018) Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. J Exp Biol 221(Pt Suppl 1). https://doi.org/10.1242/jeb.165381
Kodo K, Sugimoto S, Nakajima H et al (2017) Erythropoietin (EPO) ameliorates obesity and glucose homeostasis by promoting thermogenesis and endocrine function of classical brown adipose tissue (BAT) in diet-induced obese mice. PLoS One 12(3):e0173661. https://doi.org/10.1371/journal.pone.0173661
Romanovsky AA (2014) Skin temperature: its role in thermoregulation. Acta Physiol (Oxf) 210(3):498–507
Madden CJ, Morrison SF (2019) Central nervous system circuits that control body temperature. Neurosci Lett 696:225–232. https://doi.org/10.1016/j.neulet.2018.11.027
Meyer CW, Ootsuka Y, Romanovsky AA (2017) Body temperature measurements for metabolic phenotyping in mice. Front Physiol 8:520. https://doi.org/10.3389/fphys.2017.00520
Forni MF, Peloggia J, Braga TT et al (2017) Caloric restriction promotes structural and metabolic changes in the skin. Cell Rep 20(11):2678–2692. https://doi.org/10.1016/j.celrep.2017.08.052
Kajiya K, Kunstfeld R, Detmar M, Chung JH (2007) Reduction of lymphatic vessels in photodamaged human skin. J Dermatol Sci 47(3):241–243. https://doi.org/10.1016/j.jdermsci.2007.05.003
Kotzbeck P, Giordano A, Mondini E et al (2018) Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res 59(5):784–794. https://doi.org/10.1194/jlr.M079665
Kim DH, Gutierrez-Aguilar R, Kim HJ, Woods SC, Seeley RJ (2013) Increased adipose tissue hypoxia and capacity for angiogenesis and inflammation in young diet-sensitive C57 mice compared with diet-resistant FVB mice. Int J Obes (Lond) 37(6):853–860. https://doi.org/10.1038/ijo.2012.141
Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD (2010) Mitochondrial proton and electron leaks. Essays Biochem 47:53–67. https://doi.org/10.1042/bse0470053
Bertholet AM, Kirichok Y (2017) UCP1: a transporter for H+ and fatty acid anions. Biochimie 134:28–34. https://doi.org/10.1016/j.biochi.2016.10.013
Betz MJ, Enerback S (2018) Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol 14(2):77–87. https://doi.org/10.1038/nrendo.2017.132
Acknowledgements
Thank you to: C. Smith, M. Brunini, C. Doyle, K. O-Reilly, S. Christian, D. Castle and K. Brown (Telethon Kids Institute, Western Australia) for day-to-day care of the mice; E. Barrick (Telethon Kids Institute) for animal welfare; M. Crook (Princess Margaret Hospital Pathology, Subiaco, Western Australia) and L. Berry (Telethon Kids Institute) for help preparing the histological liver and brown adipose tissue sections; L. Gregory and A. Cheen (PathWest Laboratory at Fiona Stanley Hospital, Murdoch, Western Australia) for performing the serum cholesterol, HDL-cholesterol, LDL-cholesterol, triacylglycerol and calcium assays; M. Clarke (Centre for Metabolomics, University of Western Australia) for measuring serum 25(OH)D levels; and R. Endersby, M. Kuchibhotla and S. Gande (Telethon Kids Institute, Western Australia) for assisting with the use of the IVIS spectrum machine. The authors also gratefully acknowledge Bright Blue for their donation of the Nikon C2 Confocal Microscope, and Telethon Adventurers for their donation of the IVIS Spectrum Imaging System.
Funding
This research was supported by the Diabetes Research Foundation of Western Australia and the Telethon Kids Institute. SG is funded by an Al and Val Rosenstrauss Research Fellowship from the Rebecca L. Cooper Medical Research Foundation. RML is supported by an NHMRC Senior Research Fellowship. The study funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report, or the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
SG conceived and designed this study with input from GKD, KP, NJF, KC, MF, PHH, RML, RBW and VM. GD and KP acquired and analysed the data for the study with help from NJF, RCC, PJM and SG. TNA performed the confocal microscopy, while MKA performed the Oil Red O staining of liver samples, and MM and BOF measured serum nitrite and nitrate levels. All authors contributed towards the interpretation of findings from this study, played a role in drafting the article or revising it critically for its intellectual content, and gave their final approval for this version of the paper to be published. SG is the guarantor of this work.
Corresponding author
Ethics declarations
MF and RBW are members of the Scientific Advisory Board of AOBiome LLC, a company commercialising ammonia-oxidising bacteria for use in inflammatory skin disease. RBW is also a director of, and MF a scientific advisor for, RelaxSol Ltd, a company developing novel sunscreen and skincare products. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM
(PDF 2.09 mb)
Rights and permissions
About this article
Cite this article
Dhamrait, G.K., Panchal, K., Fleury, N.J. et al. Characterising nitric oxide-mediated metabolic benefits of low-dose ultraviolet radiation in the mouse: a focus on brown adipose tissue. Diabetologia 63, 179–193 (2020). https://doi.org/10.1007/s00125-019-05022-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-019-05022-5