Abstract
Aims/hypothesis
The aim of this study was to investigate the association between visit-to-visit variability in HbA1c and cognitive function decline in the elderly population.
Methods
We performed a pooled analysis of two prospective population-based cohorts (the Health Retirement Study [HRS] and the English Longitudinal Study of Ageing [ELSA]). Cognitive function, including memory and executive function, were assessed at baseline and every 2 years, while HbA1c levels were assessed at baseline and every 4 years. Visit-to-visit variability (VVV) in HbA1c was calculated using the CV, SD and variation independent of the mean (VIM) during the follow-up period. Linear mixed models were used to evaluate the association between HbA1c variability and cognitive function decline with adjustment for demographics, mean HbA1c, education, smoking, alcohol consumption, BMI, baseline hypertension, baseline diabetes status and HDL-cholesterol.
Results
The study enrolled 6237 participants (58.23% women, mean age 63.38 ± 8.62 years) with at least three measurements of HbA1c. The median follow-up duration was 10.56 ± 1.86 years. In the overall sample, compared with the lowest quartile of HbA1c variability, participants in the highest quartile of HbA1c variability had a significantly worse memory decline rate (−0.094 SD/year, 95% CI −0.185, −0.003) and executive function decline rate (−0.083 SD/year, 95% CI −0.125, −0.041), irrespective of mean HbA1c values over time. Among individuals without diabetes, each 1-SD increment in HbA1c CV was associated with a significantly higher rate of memory z score decline (−0.029, 95% CI −0.052, −0.005) and executive function z score decline (−0.049, 95% CI −0.079, −0.018) in the fully adjusted model.
Conclusions/interpretation
We observed a significant association between long-term HbA1c variability and cognitive decline among the non-diabetic population in this study. The effect of maintaining steady glucose control on the rate of cognitive decline merits further investigation.
Similar content being viewed by others
Introduction

Dementia is one of the most common neurodegenerative diseases worldwide [1] and results in poor quality of life among the impacted individuals and a serious public health burden for society. Cognitive decline occurs over a long period prior to dementia and is important for the monitoring and early intervention of cognitive deterioration [2]. Thus, the identification of risk factors for cognitive decline could contribute to screening individuals who are at risk of dementia.
Long-term variability in HbA1c has been proposed as an important risk factor related to mortality [3,4,5,6], cardiovascular disease (CVD) [7,8,9], nephropathy [10,11,12] and other complications [13,14,15,16,17] independent of HbA1c levels. Recently, there has been considerable interest in the emerging association between glycaemic variability and decline in cognitive function [18,19,20,21,22]. Although the previous studies mainly focused on individuals with type 2 diabetes, evidence from the general population (especially people without diabetes) is still scarce.
The Health and Retirement Study (HRS) and the English Longitudinal Study of Ageing (ELSA) were two sister cohorts that included a large and diverse population (age ≥ 50 years), and in these studies, repeated measurements of HbA1c and cognitive assessments at set time intervals were performed. In this study, we aimed to identify the relationship between visit-to-visit variability (VVV) in HbA1c and the rate of cognitive decline in two elderly populations with normal cognition at baseline. We hypothesised that a higher variability in HbA1c would be associated with accelerated cognitive decline in the elderly population.
Methods
Study population
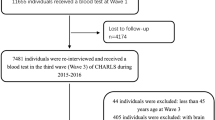
In this study, we used data from Wave 2006 to Wave 2016 of the HRS and Wave 2 (2002–2003) to Wave 8 (2014–2015) of the ELSA, two prospective and nationally representative cohorts conducted in the USA and England. Detailed descriptions of the objective, design and methods of these two cohorts have been described elsewhere [23, 24]. The time of the first HbA1c measurement was considered as the baseline for all participants. A flow chart of participant selection for the present study is shown in Fig. 1. In the HRS, a total of 3314 participants had three measurements of HbA1c from Wave 2006 to Wave 2016. Of these, 17 participants were excluded owing to lack of cognitive measurement at baseline, and 43 participants were excluded because of a history of dementia and/or Alzheimer’s disease at baseline. In the ELSA, a total of 2993 participants had three or four measurements of HbA1c from Wave 2 to Wave 8. Of these participants, ten were excluded due to lack of cognitive assessment at baseline (n = 5) or a history of dementia or Alzheimer’s disease at baseline (n = 5). The remaining participants from the HRS (n = 3254) and ELSA (n = 2983) with complete HbA1c measurements, baseline cognitive assessments and at least one reassessment of cognitive function were included in the analyses.
This study conformed to the guidelines of the Declaration of Helsinki. Institutional Review Board approval of the HRS was obtained through the University of Michigan and the National Institute on Aging. The ELSA was approved by the London Multicentre Research Ethics Committee. Informed consent was obtained from all participants in both cohorts.
Measurement of HbA1c and HbA1c variability
Blood sample collection and measurement of HbA1c in the HRS and ELSA were conducted every 4 years. Details of the process have been described elsewhere [25, 26]. National Health and Nutrition Examination Survey (NHANES)-equivalent assay values of HbA1c in the HRS were used in our study as recommended [26]. In this study, HbA1c was reported as the percentage of the erythrocyte haemoglobin that was glycated.
The mean HbA1c value was calculated based on the mean of all visits for each participant. The VVV in HbA1c was primarily defined as the intra-individual CV across visits. Due to the lack of an appropriate gold-standard measurement for HbA1c VVV, we calculated two other metrics, including the SD and the variation independent of the mean (VIM). These calculation processes have been previously described [27].
Measurement of cognitive function
Cognitive function was assessed every 2 years in both the HRS and ELSA using a variety of tests, including self-rated memory, immediate and delayed word recall, and backward count. In this study, we used scores of memory test and executive function as outcomes, which were assessed in both the HRS and ELSA. We created a total memory recall score ranging from 0 to 20 by summing the scores of immediate and delayed recall tests. A higher score on the memory recall test indicated better memory performance. Executive function was assessed by a verbal fluency task in which participants were required to orally list as many animal names as they could in 60 s. The score of verbal fluency was calculated as the total count of words excluding repeated and non-animal words, and higher scores indicated better executive function. Both immediate and delayed word recall tests and verbal fluency tests have been shown to have good validity and consistency [28, 29].
The standardised z score for the cognitive test scores at each wave was calculated by subtracting the mean score at baseline and dividing the value by the SD of the baseline scores. Thus, a z score of 1 means the performance on the particular cognitive test was 1 SD above the mean score at baseline.
Covariates
The covariates included demographic and clinical variables. The demographic variables included age, sex, educational level (college or above), BMI, living arrangement (living alone or not), current cigarette smoking, and current alcohol consumption (alcohol consumption at least 1 day per week). Clinical variables included history or presence of CVD (myocardial infarction, CHD, revascularisation, stroke and peripheral arterial disease of heart failure), hypertension, diabetes, lung disease and cancer. Depressive symptoms were measured using an 8-item version of the Center for Epidemiologic Studies Depression Scale (CES-D). Mean systolic BP across visits was also calculated for each participant. Baseline HDL, tested in both the HRS and ELSA, was also included.
Statistical analysis
All participants were categorised into quartiles of CV of HbA1c. Categorical variables are presented as numbers (proportions), and continuous variables are presented as the mean ± SD. We used the Cochran–Armitage trend test for categorical variables and linear regression for continuous variables to test the significance of the trends across the quartiles.
We used a multivariable linear mixed-effect model (a widely used model to address repeated measurement data) to evaluate the longitudinal association between VVV in HbA1c and cognitive decline. In the current study, the intercept and slope were both fitted as random effects to address the inter-individual differences at baseline and different rates of cognitive function change during the follow-up period. A negative β value for the interaction item of time and CV of HbA1c indicated that a 1-unit increment of CV of HbA1c was associated with a faster rate of decline with increasing time in the study. Two models were used as follows: Model I included the CV of HbA1c (the lowest quartile as the reference group), time (years from baseline), interaction item of time and CV of HbA1c, mean HbA1c value, interaction item of time and mean HbA1c value, age (continuous variable) and sex (male or female). Model II included all covariates in Model I and was additionally adjusted for education, current smoking status (yes or no), alcohol consumption status (yes or no), BMI (continuous variable), hypertension (yes or no), diabetes (yes or no) and HDL (continuous variable). The p value for the trend was calculated by using the median value in each quartile of the HbA1c CV. The effect of each SD increment in HbA1c CV was also calculated by modelling HbA1c CV as a continuous variable. Subgroup analysis was conducted by stratifying the participants into two groups based on their status of diabetes at baseline: diabetes and non-diabetes. The effects were calculated within each cohort separately and were pooled using a random-effects model. Heterogeneity of β values between the two cohorts was evaluated by the Cochran’s Q test and I2 statistic.
The multiple imputation of chained equations (MICEs) method was also used to impute missing data from the cognitive assessments during follow-up. Baseline characteristics, including age, sex, education, BMI, smoking status, alcohol consumption status, diabetes, hypertension and baseline cognitive scores, were used to impute the missing values. We created 20 imputed datasets and pooled the results using the R package ‘MICE’ for each model. The imputation quality was assessed by comparing the imputed data with the original data using density plots. Sensitivity analyses were conducted as follows: (1) modelling SD or VIM instead of CV as VVV metrics of HbA1c; (2) repeating analysis with all available data without multiple imputations; (3) removing the participants with three HbA1c measurements in the ELSA from the analysis.
All statistical analyses were performed using R software 3.4.1 (R Foundation, Vienna, Austria). The R package ‘lme4’ was used to perform the linear mixed-effect model [30]. Two-sided p values less than 0.05 were considered statistically significant.
Results
Baseline characteristics
A total of 6237 participants (3254 from the HRS and 2983 from the ELSA) were included in the analysis. The mean age at baseline was 64.55 ± 9.11 years in the HRS and 62.03 ± 7.79 years in the ELSA. A total of 1974 (60.66%) participants in the HRS and 1658 (55.58%) participants in the ELSA were female. The mean follow-up times were 10.48 ± 0.63 years and 10.56 ± 1.86 years for the HRS and ELSA, respectively. A comparison of baseline characteristics between participants included and not included is shown in electronic supplementary material (ESM) Table 1.
Table 1 presents the characteristics of the included participants across quartiles of HbA1c CV. Participants in the highest quartile of HbA1c CV had a higher BMI, baseline HbA1c, mean HbA1c across visits, mean systolic BP, prevalence of hypertension and diabetes, and a lower education level and HDL in both the HRS and ELSA. Participants tended to have a lower baseline cognitive function (both total recall score and verbal fluency score) as the quartiles of HbA1c CV increased (p for trend <0.001).
Association of HbA1c variability with cognitive decline in the overall sample
As listed in Tables 2 and 3, a 1% increase in the mean HbA1c value was associated with an increased rate of decline in the memory z score (−0.089 SD/year, 95% CI −0.165, −0.014) and executive function z score (−0.058 SD/year, 95% CI −0.111, −0.004) in Model I. This association was attenuated after further adjustment for covariates in Model II but remained significant for memory function (−0.041, 95% CI −0.071, −0.012).
We observed a potential dose–response relationship between the quartiles of HbA1c CV and the rate of cognitive decline. Participants in the highest quartile had a significantly accelerated rate of memory z score decline (−0.100 SD/year, 95% CI −0.197, −0.001) and executive function z score decline (−0.081 SD/year, 95% CI −0.126, −0.037) compared with that of the lowest quartile of HbA1c CV (p for trend <0.05). The results remained significant when further adjusted for education level, baseline BMI, smoking status, alcohol consumption status, disease status at baseline (including hypertension and diabetes) and HDL at baseline (ESM Fig. 1). When modelled as a continuous variable, a 1-SD increment in HbA1c CV was associated with both a higher rate of memory z score decline (−0.032 SD/year, 95% CI −0.049, −0.015) and executive function z score decline (−0.034 SD/year, 95% CI −0.084, −0.010) in the fully adjusted models. We did not observe the effect modification by sex in the current study (ESM Fig. 2).
HbA1c variability and cognitive decline among participants with diabetes
For diabetic participants (Fig. 2), each 1-SD increment in the CV of HbA1c was not associated with an increased rate of memory z score decline (−0.028 SD/year, 95% CI −0.065, 0.008) or executive function z score decline (0.018 SD/year, 95% CI −0.029, 0.064) (Model II). When modelled as a categorical variable (ESM Table 2), the highest quartile of HbA1c CV was associated with neither memory function (−0.023 SD/year, 95% CI −0.129, 0.082) nor executive function (0.064 SD/year, 95% CI −0.142, 0.270) in the fully adjusted model compared with the lowest quartile.
HbA1c variability and cognitive decline among participants without diabetes
For non-diabetic participants at baseline, each 1-SD increment in HbA1c CV was associated with a significantly higher rate of memory z score decline (−0.029 SD/year, 95% CI −0.052, −0.005) and executive function z score decline (−0.049 SD/year, 95% CI −0.079, −0.018) in the fully adjusted model. When the CV of HbA1c was modelled as a categorical variable, compared with the lowest quartile, the highest quartile of the CV of HbA1c was associated with a higher rate of memory decline (−0.055 SD/year, 95% CI −0.096, −0.013) and executive function decline (−0.088 SD/year, 95% CI −0.197, −0.022) in the fully adjusted model (ESM Table 2).
Sensitivity analysis
A similar pattern except for the significance of trend across quartiles of HbA1c CV was observed in a sensitivity analysis by excluding participants with missing values of cognitive assessment (ESM Table 3). ESM Fig. 3 shows that the distribution of the imputed data is generally similar to that of the original data. Sensitivity analyses by using the SD and VIM as indices of HbA1c variability did not substantially alter our findings (ESM Tables 4 and 5), but p values for the trend were not consistent with the main analysis. When we restricted the participants to those with four HbA1c measurements in the ELSA, a positive association between HbA1c variability and memory and executive function was still observed (ESM Table 6).
Discussion
In these two large population-based prospective cohorts (the HRS and ELSA) with a mean follow-up period of 10.48 years, we examined the association between HbA1c variability and cognitive decline. Overall, we observed that greater HbA1c variability was associated with steeper decline in cognitive function independent of mean HbA1c values among individuals without diabetes but not among individuals with diabetes. Sensitivity analyses did not materially change our results. Our findings could provide evidence of the detrimental effect of HbA1c variability and highlight the significance of steady glycaemic control.
To the best of our knowledge, this is the first study of the association between long-term HbA1c variability and cognitive decline that analyses data from more than three cognitive function measurements over time. Moreover, prior epidemiological and clinical studies on this topic were mainly focused on individuals with type 2 diabetes mellitus [21, 22, 31, 32]. Two cross-sectional studies, both conducted among individuals with type 2 diabetes, reported significantly worse cognitive performance among participants with a greater degree of visit-to-visit glucose variability. Our study further extended the findings of a significant association between HbA1c VVV and cognitive decline among a non-diabetic population. Using data from two large prospective cohorts with biennial repeated cognitive measurements and a 10-year period of total follow-up, we were able to calculate the long-term glycaemic variability and trajectory of cognitive decline and investigate the association. Prior mechanics studies [33,34,35] also suggested a possible deleterious effect of glycaemic variability among individuals without diabetes, which was confirmed by our findings. Bancks et al found that higher intra-individual fasting glucose variability during young adulthood was associated with worse cognitive performances in midlife, and this association was stronger among individuals without diabetes [36]. These findings were consistent with the present result, although the cognitive tests used were different from those used in the current study. Positive associations were also observed between long-term glycaemic variability and mortality, CVD and type 2 diabetes [7, 37,38,39] among the non-diabetic population, which aligns with our findings.
The lack of a significant association of HbA1c variability with cognitive function decline among individuals with diabetes may be due to a number of factors. Bancks et al [36] assessed glucose variation before diabetes onset in the CARDIA study and observed that fasting glucose CV was more strongly associated with worse cognitive test scores among individuals without diabetes at the time of the cognitive test than among individuals with diabetes. This suggested that medication use for diabetes may disrupt the natural course of glycaemic variation and blunt the association of HbA1c variability with cognitive function. The relatively small number of participants with diabetes in the present study (n = 189 in the ELSA and n = 651 in the HRS) may restrict the power to detect a positive association. Moreover, previous post hoc analysis of clinical trials showed that significant associations between long-term glycaemic variation and outcomes (CVD or mortality) were only observed in the intensive glucose-lowering arm but not in the standard glucose-lowering arm [8, 40]. The latter is somehow consistent with our results, as elderly people with diabetes are unlikely to receive intensive glucose-lowering treatment according to the recommendations of the ADA [41]. Several studies have also reported that long-term HbA1c variability has a greater impact among individuals without diabetes, while short-term variability is a predictor among those with diabetes [33, 42]. Future studies are still needed to verify these observed associations.
Our findings also have implications for clinical practice. Individuals with large variability in HbA1c levels, although with absolute measurement level within the normal range, may have been neglected in regard to their risk of cognitive decline. Therefore, attention should be paid to the homeostasis of HbA1c levels in older adults. Measuring long-term HbA1c variability might help predict cognitive decline among individuals without diabetes. However, the causal relationship warrants verification in large clinical trials, and strategies to both maintain HbA1c absolute levels and reduce HbA1c variability merit further investigation.
The exact mechanism of the detrimental effect of glycaemic variability on cognitive decline remains unknown. Excessive insulin secretion caused by hyperglycaemia may result in peripheral or cerebral insulin resistance associated with neuronal vulnerability, neurodegeneration and further pathological lesions [43]. Impairment of insulin receptors and signalling in the brain may affect neuronal survival, astrocyte inflammatory cytokine secretion, nitric oxide-mediated vasodilation and cerebral perfusion [43]. A number of studies have indicated that glycaemic variability may cause apoptosis of pancreatic beta cells, which could result in the deterioration of glycaemic control and subsequent diabetic complications [44, 45].
Our study has several strengths, including the large population-based sample size, the inclusion of several metrics of HbA1c variability, the adoption of reliable tests of cognitive function, the appropriate statistical model and adjustment for mean HbA1c values. However, our findings should be interpreted in the context of the following limitations. First, because of the observational design, only associations, not causality, can be inferred from our results. Second, large intervals between HbA1c measurements (4 years) and relatively few measurements may restrict the generalisability of our findings. Third, although the HRS and ELSA were sister cohorts with a similar design, there are significant heterogeneities between the two sides of the Atlantic. Therefore, we used a meta-analysis approach with the random-effect model to combine the results from the two cohorts. Additionally, we excluded participants with less than three values of HbA1c in our study, but their characteristics were different from the included participants: the excluded participants were older, included a higher percentage of smokers, and had more depressive symptoms (ESM Table 1). Therefore, some caution is still necessary when extrapolating our findings to other populations. Fourth, we were unable to examine the relationship between long-term HbA1c VVV and the incidence of dementia/Alzheimer’s disease due to the lack of rigorous clinical diagnoses of dementia or Alzheimer’s disease during the follow-up of the HRS and ELSA. Finally, although we adjusted for several potential confounders, unmeasured variables such as genetic susceptibility, data for which were not available, may have affected our results.
Conclusion
In summary, our study provides evidence to support the association between long-term HbA1c variability and cognitive decline irrespective of the effect of mean HbA1c values among individuals without diabetes. Further studies are needed to determine whether decreasing glycaemic variability would benefit cognitive decline in the elderly.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CVD:
-
Cardiovascular disease
- ELSA:
-
English Longitudinal Study of Ageing
- HRS:
-
Health and Retirement Study
- MICE:
-
Multiple imputation of chained equation
- VIM:
-
Variation independent of the mean
- VVV:
-
Visit-to-visit variability
References
Livingston G, Sommerlad A, Orgeta V et al (2017) Dementia prevention, intervention, and care. Lancet 390(10113):2673–2734. https://doi.org/10.1016/S0140-6736(17)31363-6
Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA (2014) Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol 2(3):246–255. https://doi.org/10.1016/S2213-8587(13)70088-3
Forbes A, Murrells T, Mulnier H, Sinclair AJ (2018) Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol 6(6):476–486. https://doi.org/10.1016/S2213-8587(18)30048-2
Orsi E, Solini A, Bonora E et al (2018) Haemoglobin A1c variability is a strong, independent predictor of all-cause mortality in patients with type 2 diabetes. Diabetes Obes Metab 20(8):1885–1893. https://doi.org/10.1111/dom.13306
Wan EY, Fung CS, Fong DY, Lam CL (2016) Association of variability in hemoglobin A1c with cardiovascular diseases and mortality in Chinese patients with type 2 diabetes mellitus - a retrospective population-based cohort study. J Diabetes Complicat 30(7):1240–1247. https://doi.org/10.1016/j.jdiacomp.2016.05.024
Xu D, Fang H, Xu W, Yan Y, Liu Y, Yao B (2016) Fasting plasma glucose variability and all-cause mortality among type 2 diabetes patients: a dynamic cohort study in Shanghai. China. Sci Rep 6(1):39633. https://doi.org/10.1038/srep39633
Echouffo-Tcheugui JB, Zhao S, Brock G, Matsouaka RA, Kline D, Joseph JJ (2019) Visit-to-visit glycemic variability and risks of cardiovascular events and all-cause mortality: the ALLHAT study. Diabetes Care 42(3):486–493. https://doi.org/10.2337/dc18-1430
Hirakawa Y, Arima H, Zoungas S et al (2014) Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care 37(8):2359–2365. https://doi.org/10.2337/dc14-0199
Waden J, Forsblom C, Thorn LM et al (2009) A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 58(11):2649–2655. https://doi.org/10.2337/db09-0693
Jun JE, Lee SE, Lee YB et al (2017) Glycated albumin and its variability as an indicator of cardiovascular autonomic neuropathy development in type 2 diabetic patients. Cardiovasc Diabetol 16(1):127. https://doi.org/10.1186/s12933-017-0619-2
Nazim J, Fendler W, Starzyk J (2014) Metabolic control and its variability are major risk factors for microalbuminuria in children with type 1 diabetes. Endokrynol Pol 65(2):83–89. https://doi.org/10.5603/EP.2014.0012
Rodriguez-Segade S, Rodriguez J, Garcia Lopez JM, Casanueva FF, Camina F (2012) Intrapersonal HbA(1c) variability and the risk of progression of nephropathy in patients with type 2 diabetes. Diabet Med 29(12):1562–1566. https://doi.org/10.1111/j.1464-5491.2012.03767.x
Kilpatrick ES, Rigby AS, Atkin SL (2008) A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care 31(11):2198–2202. https://doi.org/10.2337/dc08-0864
Hietala K, Waden J, Forsblom C et al (2013) HbA1c variability is associated with an increased risk of retinopathy requiring laser treatment in type 1 diabetes. Diabetologia 56(4):737–745. https://doi.org/10.1007/s00125-012-2816-6
Hermann JM, Hammes HP, Rami-Merhar B et al (2014) HbA1c variability as an independent risk factor for diabetic retinopathy in type 1 diabetes: a German/Austrian multicenter analysis on 35,891 patients. PLoS One 9(3):e91137. https://doi.org/10.1371/journal.pone.0091137
Ravona-Springer R, Heymann A, Schmeidler J et al (2017) Hemoglobin A1c variability predicts symptoms of depression in elderly individuals with type 2 diabetes. Diabetes Care 40(9):1187–1193. https://doi.org/10.2337/dc16-2754
Yang CP, Li CI, Liu CS et al (2017) Variability of fasting plasma glucose increased risks of diabetic polyneuropathy in T2DM. Neurology 88(10):944–951. https://doi.org/10.1212/wnl.0000000000003682
Rawlings AM, Sharrett AR, Mosley TH, Ballew SH, Deal JA, Selvin E (2017) Glucose peaks and the risk of dementia and 20-year cognitive decline. Diabetes Care 40(7):879–886. https://doi.org/10.2337/dc16-2203
Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ (2015) Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 3(1):75–89. https://doi.org/10.1016/S2213-8587(14)70148-2
Cukierman-Yaffe T, Gerstein HC, Williamson JD et al (2009) Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 32(2):221–226. https://doi.org/10.2337/dc08-1153
Kim C, Sohn JH, Jang MU et al (2015) Association between visit-to-visit glucose variability and cognitive function in aged type 2 diabetic patients: a cross-sectional study. PLoS One 10(7):e0132118. https://doi.org/10.1371/journal.pone.0132118
Cui X, Abduljalil A, Manor BD, Peng CK, Novak V (2014) Multi-scale glycemic variability: a link to gray matter atrophy and cognitive decline in type 2 diabetes. PLoS One 9(1):e86284. https://doi.org/10.1371/journal.pone.0086284
Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR (2014) Cohort profile: the health and retirement study (HRS). Int J Epidemiol 43(2):576–585. https://doi.org/10.1093/ije/dyu067
Steptoe A, Breeze E, Banks J, Nazroo J (2013) Cohort profile: the English longitudinal study of ageing. Int J Epidemiol 42(6):1640–1648. https://doi.org/10.1093/ije/dys168
Graig R, Deverill C, Pickering K (2006) Quality control of blood saliva and urine analytes. In: Spronston K, Mindell J (eds) Health Survey for England 2004: methodology and documentation, vol 2. The Information Centre, London
Eileen MC, Jessica DF, Jung Ki K et al (2013) Documentation of biomarkers in the 2006 and 2008 health and retirement study. Institute for Social Research, University of Michigan, Ann Arbor, Michigan
Rothwell PM (2010) Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 375(9718):938–948. https://doi.org/10.1016/S0140-6736(10)60309-1
Baars MA, van Boxtel MP, Dijkstra JB et al (2009) Predictive value of mild cognitive impairment for dementia. The influence of case definition and age. Dement Geriatr Cogn Disord 27(2):173–181. https://doi.org/10.1159/000200465
Dregan A, Stewart R, Gulliford MC (2013) Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing 42(3):338–345. https://doi.org/10.1093/ageing/afs166
Bates D, Machler M, Bolker WS (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Li TC, Yang CP, Tseng ST et al (2017) Visit-to-visit variations in fasting plasma glucose and HbA1c associated with an increased risk of Alzheimer disease: Taiwan diabetes study. Diabetes Care 40(9):1210–1217. https://doi.org/10.2337/dc16-2238
Rizzo MR, Marfella R, Barbieri M et al (2010) Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care 33(10):2169–2174. https://doi.org/10.2337/dc10-0389
Ceriello A, Esposito K, Piconi L et al (2008) Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57(5):1349–1354. https://doi.org/10.2337/db08-0063
Salkind SJ, Huizenga R, Fonda SJ, Walker MS, Vigersky RA (2014) Glycemic variability in nondiabetic morbidly obese persons: results of an observational study and review of the literature. J Diabetes Sci Technol 8(5):1042–1047. https://doi.org/10.1177/1932296814537039
Hanefeld M, Sulk S, Helbig M, Thomas A, Köhler C (2014) Differences in glycemic variability between normoglycemic and prediabetic subjects. J Diabetes Sci Technol 8(2):286–290. https://doi.org/10.1177/1932296814522739
Bancks MP, Carnethon MR, Jacobs DR Jr et al (2018) Fasting glucose variability in young adulthood and cognitive function in middle age: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Diabetes Care 41(12):2579–2585. https://doi.org/10.2337/dc18-1287
Wang A, Liu X, Xu J, Han X et al (2017) Visit-to-visit variability of fasting plasma glucose and the risk of cardiovascular disease and all-cause mortality in the general population. J Am Heart Assoc 6(12). https://doi.org/10.1161/JAHA.117.006757
Ghouse J, Skov MW, Kanters JK et al (2019) Visit-to-visit variability of hemoglobin a in people without diabetes and risk of major adverse cardiovascular events and all-cause mortality. Diabetes Care 42(1):134–141. https://doi.org/10.2337/dc18-1396
Kim JA, Lee JS, Chung HS et al (2018) Impact of visit-to-visit fasting plasma glucose variability on the development of type 2 diabetes: a nationwide population-based cohort study. Diabetes Care 41(12):2610–2616. https://doi.org/10.2337/dc18-0802
Zhou JJ, Schwenke DC, Bahn G, Reaven P (2018) Glycemic variation and cardiovascular risk in the Veterans Affairs Diabetes Trial. Diabetes Care 41(10):2187–2194. https://doi.org/10.2337/dc18-0548
Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Mañas L (2011) European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 37(Suppl 3):S27–S38. https://doi.org/10.1016/s1262-3636(11)70962-4
Monnier L, Mas E, Ginet C et al (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295(14):1681–1687. https://doi.org/10.1001/jama.295.14.1681
Arnold SE, Arvanitakis Z, Macauley-Rambach SL et al (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 14(3):168–181. https://doi.org/10.1038/nrneurol.2017.185
Del Guerra S, Grupillo M, Masini M et al (2007) Gliclazide protects human islet beta-cells from apoptosis induced by intermittent high glucose. Diabetes Metab Res Rev 23(3):234–238. https://doi.org/10.1002/dmrr.680
U.K. Prospective Diabetes Study Group (1995) U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 44(11):1249–1258. https://doi.org/10.2337/diab.44.11.1249
Acknowledgements
We thank the University of Michigan for the use of data from the HRS Waves 8–13 from 2006–2016 and the UK Data Archive for the use of data from the ELSA Waves 2–7 from 2002–2015. The original data creators, depositors or copyright holders bear no responsibility for the current analysis or interpretation.
Funding
The HRS data are sponsored by the National Institute on Aging (grant number U01AG009740), and the study is being conducted by the University of Michigan. The current study was funded by the Ministry of Science and Technology of the People’s Republic of China (No. 2015FY111600). The funders played no role in the study design, data collection, analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
KC is the guarantor of this work and had full access to all of the data in the study. KC and ZY conceptualised the study and designed the analysis plan. ZY performed all the statistical analyses and drafted the manuscript. JW provided supervision to ZY. All authors contributed to the acquisition, analysis or interpretation of data; provided critical revision of the manuscript for important intellectual content and approved the final version. KC is responsible for the integrity of the work as a whole.
Corresponding authors
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM
(PDF 463 kb)
Rights and permissions
About this article
Cite this article
Yu, ZB., Zhu, Y., Li, D. et al. Association between visit-to-visit variability of HbA1c and cognitive decline: a pooled analysis of two prospective population-based cohorts. Diabetologia 63, 85–94 (2020). https://doi.org/10.1007/s00125-019-04986-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-019-04986-8