Abstract
Aims/hypothesis
Bi-allelic loss-of-function mutations in the INSR gene (encoding the insulin receptor [INSR]) commonly cause extreme insulin resistance and early mortality. Therapeutic options are limited, but anti-INSR antibodies have been shown to activate two mutant receptors, S323L and F382V. This study evaluates four well-characterised murine anti-INSR monoclonal antibodies recognising distinct epitopes (83-7, 83-14, 18-44, 18-146) as surrogate agonists for potential targeted treatment of severe insulin resistance arising from insulin receptoropathies.
Methods
Ten naturally occurring mutant human INSRs with defects affecting different aspects of receptor function were modelled and assessed for response to insulin and anti-INSR antibodies. A novel 3T3-L1 adipocyte model of insulin receptoropathy was generated, permitting conditional knockdown of endogenous mouse Insr by lentiviral expression of species-specific short hairpin (sh)RNAs with simultaneous expression of human mutant INSR transgenes.
Results
All expressed mutant INSR bound to all antibodies tested. Eight mutants showed antibody-induced autophosphorylation, while co-treatment with antibody and insulin increased maximal phosphorylation compared with insulin alone. After knockdown of mouse Insr and expression of mutant INSR in 3T3-L1 adipocytes, two antibodies (83-7 and 83-14) activated signalling via protein kinase B (Akt) preferentially over signalling via extracellular signal-regulated kinase 1/2 (ERK1/2) for seven mutants. These antibodies stimulated glucose uptake via P193L, S323L, F382V and D707A mutant INSRs, with antibody response greater than insulin response for D707A.
Conclusions/interpretation
Anti-INSR monoclonal antibodies can activate selected naturally occurring mutant human insulin receptors, bringing closer the prospect of novel therapy for severe insulin resistance caused by recessive mutations.
Similar content being viewed by others

Introduction
Insulin downregulates catabolic and activates anabolic pathways, suppresses apoptosis and promotes mitosis by activating a homodimeric receptor, tyrosine kinase [1, 2]. Human loss-of-function mutations in the INSR gene, which encodes the insulin receptor (INSR), were first reported in 1988 [3, 4]. Since then, more than 100 alleles causing severe insulin resistance have been described [5]. Bi-allelic INSR mutations produce extreme insulin resistance, clinically described as Donohue or Rabson–Mendenhall syndromes (OMIM #246200 or #262190). These also feature impaired linear growth and soft tissue overgrowth, with demise usually in the first 3 years of life in Donohue syndrome.
Some INSR mutations impair receptor processing and cell surface expression. Many mutations, however, are well expressed, but exhibit impaired insulin binding, impaired signal transduction, perturbed recycling kinetics or a combination of these [6]. Proof that the signalling defect of such mutant receptors might be circumvented by binding anti-receptor antibodies was provided for two mutations, one in a cell culture model and one as solubilised receptor [7, 8].
Therapeutic antibodies are now well established both in cancer, often blocking receptor signalling [9], and increasingly for non-cancer indications [10]. Interest in biological therapies targeting the INSR has recently rekindled, with inhibitory antibodies in Phase 1 human trials [11] and stimulatory antibodies shown to ameliorate diabetes in rodents [12,13,14] and primates [15]. Given the high clinical need in recessive insulin receptoropathy, we assessed the effect of monoclonal anti-INSR antibodies [16,17,18,19,20] on a series of disease-causing mutant INSRs.
Methods
Cell lines and culture conditions
Culture media for Chinese hamster ovary (CHO) Flp-In cells (Invitrogen, Carlsbad, CA, USA) and 3T3-L1 pre-adipocytes (Zenbio, Raleigh, NC, USA) are shown in electronic supplementary (ESM) Table 1. Cell lines were all mycoplasma negative by PCR. 3T3-L1 pre-adipocytes were grown to confluence and differentiation was induced by differentiation medium 1 for 72 h then differentiation medium 2 for a further 72 h. Adipocytes were maintained in adipocyte medium containing 1 μmol/l insulin ±1 μg/ml doxycycline (DOX). Experiments were undertaken at day 14 or 16 of differentiation.
hINSR mutant expression constructs and generation of CHO Flp-In hINSR cells
Mutation numbering refers to mature hINSR ex11+ (GenBank M1005.1), which was amplified from pDNR-Dual (Clontech, Mountain View, CA, USA) using primers incorporating a C-terminus myc-tag. Sub-cloning is detailed in ESM Table 2. Mutations were generated with the Quickchange II XL kit (Stratagene, La Jolla, CA, USA). CHO Flp-In cells were transfected with pCDNA5/FRT/TO/hINSR and pOG44 using Lipofectamine 2000 (Invitrogen). The population surviving hygromycin B was used for experiments.
Lentivirus production and infection of 3T3-L1 pre-adipocytes
Target sequences, primers, vectors and sub-cloning steps are detailed in ESM Table 3. Virus was packaged and concentrated as described by Shin et al [21]. 3T3-L1 pre-adipocytes were infected with the lowest multiplicity of infection (MOI) of virus needed to confer hygromycin B resistance. Several clones per line were characterised for endogenous Insr knockdown and adipocyte differentiation by Oil Red O staining [22]. For hINSR re-expression studies, 3T3-L1 murine Insr-knockdown (MmINSRKD) cells were infected with virus containing myc-tagged hINSR transgenes at the lowest MOI needed to confer G418 resistance to generate polyclonal populations. hINSR expression was confirmed by cDNA sequencing.
Flow cytometry
CHO Flp-In hINSR cells were blocked by 5% (vol./vol.) FCS/FACS buffer (ESM Table 4) before incubation with primary antibodies for 1 h at 4°C. Bound antibodies were detected using FITC-conjugated anti-mouse IgG and a BD FACSCalibur Flow Cytometer (530 nm/30 nm bandwidth filter, Becton Dickinson, Franklin Lakes, NJ, USA). Stacked histograms were visualised with FCS Express 6 Plus (DeNovo Software, Glendale, CA, USA).
Receptor autophosphorylation assays
CHO Flp-In hINSR cells were washed twice and serum starved (16 h) before stimulation with insulin, antibody or both for 10 min at 37°C/5% CO2 and lysed on ice in lysis buffer (ESM Table 4). Receptors were captured overnight at 4°C on anti-myc antibody 9E10-coated white Greiner Lumitrac 600 96 well plates. Phosphotyrosines on immunocaptured receptors were detected with biotin-conjugated 4G10 platinum phospho-tyrosine antibody and europium-labelled streptavidin. DELFIA enhancement solution was added and time-resolved fluorescence measured (excitation 340 nm/emission 615 nm).
Downstream signal activation
3T3-L1 adipocytes were washed twice in DMEM, serum starved for 16 h in DMEM/0.5% BSA/1 μg/ml DOX and treated for 10 min at 37°C/5% CO2 with 10 nmol/l insulin, 10 nmol/l antibody or both in DMEM/0.5% (wt/vol.) BSA. Cells were washed, snap frozen and lysed on ice before centrifugation twice at 4°C for 15 min to pellet insoluble material and separate lipids prior to western blotting.
Western blotting
Lysate, 10 μg, was resolved on NuPAGE 4–12% bis-tris gels or E-PAGE 48 8% gels (Life Technologies, Carlsbad, CA, USA) and transferred to nitrocellulose by iBlot (Life Technologies). Membranes were blocked in 3% BSA (wt/vol.)/tris-buffered saline with Tween 20 (TBST) before overnight incubation at 4°C with primary antibodies (ESM Table 5). Horseradish peroxidase (HRP)-conjugated secondary antibodies and Immobilon Western Chemiluminescent HRP substrate (Millipore, Darmstadt, Germany) were used to detect protein–antibody complexes, and grey-scale 16 bit tag image file formats (TIFFs) captured with an ImageQuant LAS4000 camera system (GE Healthcare Lifesciences, Marlborough, MA, USA). Each immunoblot in Fig. 4 and ESM Fig. 2 contained a sample of 3T3-L1 MmINSRKD hINSR wild type (WT) treated with 10 nmol/l insulin.
Western blot image densitometry
Pixel density of grey-scale 16 bit TIFFs was determined in ImageJ 1.47v (NIH, Bethesda, MD, USA). The rectangle tool was used to select lanes and the line tool to enclose the peak of interest and subtract background. The magic-wand tool was used to select the peak area and obtain the raw densitometry value. Mean band intensities of total INSRβ, myc-tagged INSRβ, Akt, extracellular signal-regulated kinase 1/2 (ERK1/2), glycogen synthase kinase 3 (GSK3)α/β, ribosomal protein S6 kinase β 1 (p70S6K) and calnexin were used to normalise raw densitometry values for p-INSRβ, p-Akt, p-ERK1/2, p-GSK3α, p-p70S6K and p-Akt substrate of 160 kDa (p-AS160). Normalised values for phosphorylated targets were scaled to the mean WT INSR response to insulin.
Glucose uptake
3T3-L1 adipocytes were washed twice (DMEM), serum starved for 16 h in low-glucose DMEM/0.2% BSA/1 μg/ml DOX, washed twice in PBS and then stimulated for 30 min at 37°C/5% CO2 with 10 nmol/l insulin, 10 nmol/l antibody or both in KRPH/0.2% BSA buffer (ESM Table 4). Cells were incubated with 1 mmol/l 2-deoxy-d-glucose for 5 min at 37°C/5% CO2 before washing (PBS), lysing with 0.1 mol/l NaOH, and snap freezing. Glucose uptake was measured by the fluorescence method of Yamamoto et al [23].
Statistical analysis
One-way ANOVAs with Tukey’s multiple comparisons test were performed with GraphPad Prism 6 (GraphPad software, San Diego, CA, USA). Error bars represent SEM or SD as indicated. All experiments were performed at least three times.
Results
Assessment of mutant INSR cell surface expression and antibody binding
Eleven INSR mutations were selected for study (ESM Table 6). Eight were chosen based on evidence of cell surface expression, prioritising mutations identified in multiple reports to maximise potential availability of participants for future trials. A previously unpublished mutation, F248C, that we identified in a child with Rabson–Mendenhall syndrome, was included opportunistically. The well-studied P1178L tyrosine kinase mutation [24, 25] and the L62P mutation, which severely impairs processing [26], were added as controls. Figure 1a displays the extracellular INSR mutations mapped onto the crystal structure of the INSR [27]. Four mouse monoclonal anti-human INSR antibodies were used, which had all previously been shown to have partial agonist activity at WT receptors, but different effects on kinetics and affinity of insulin binding (Table 1). As Fab fragments of 83-7 and 83-14 were used in determining the crystal structure of insulin-bound INSR [28], their binding epitopes are known (Fig. 1b).
Mutant INSR is expressed at the cell surface and bound by anti-INSR antibody. (a) INSR monomer Protein Data Bank (PDB) structure entry 4ZXB [22] visualised with CCP4MG (v. 2.10.6); locations of mutated residues (this study) are highlighted in red. (b) INSR monomer in complex with Fab fragments 83-7 and 83-14, PDB structure 4ZXB [22]. (c) Western blot of lysates from CHO Flp-In cells stably expressing human WT or mutant INSR, as indicated. In INSRβ subunit and myc-tag blots, upper bands are pro-INSR and lower bands are mature processed β subunits, as indicated. (d–g) Stacked overlay single parameter histograms showing cell surface expression of INSR mutants bound by antibodies 83-7 (d), 83-14 (e), 18-44 (f) and 18-146 (g), as determined by flow cytometry. Intensity of INSR-FITC fluorescence is shown on the x-axis and the peak height indicates relative number of events. Isotype control IgG (light grey) was used as a negative control to generate a negative gate to determine the percentage of the population positive for anti-INSR antibody binding. Rightward shift of the peak (blue 83-7, cyan 83-14, orange 18-44, purple 18-146) from the IgG control is a function of both mutant INSR expression and antibody affinity. Pos, positive
Mutations were introduced into the B isoform of the INSR, believed to be the more important isoform for the metabolic actions of insulin [29]. To enable discrimination of endogenous INSR and human INSR mutants, a C-terminal myc-tag was used in the mutant constructs. Tagged mutants were expressed in CHO cells using the Flp-In system, ensuring differences in protein expression are due to differential processing or stability of receptor protein rather than differential mRNA expression. The mutants were well processed to mature β subunits, with the exception of L62P, for which β subunit was barely detectable. More modest reductions were seen for the previously unstudied F248C and for the P1178L mutation (Fig. 1c).
Cell surface expression and antibody binding of mutant INSR was assessed by flow cytometry (Fig. 1d–g). All INSR antibodies bound each mutant INSR, as shown by right-shifted peaks relative to control IgG, indicating no gross changes in receptor morphology. Poor expression of L62P was in keeping with prior reports [30], and L62P was not studied further. The rightward shift for mutants corresponded to expression of mature β subunits seen by immunoblotting, suggesting that relative shifts reflected differences in receptor expression rather than antibody affinities. Although some mutations are close to the epitope for antibody 83-7, none of the affected residues provides critical antibody contacts. Indeed, no difference in binding of 83-7 (Fig. 1d) to the mutant panel was seen compared with 83-14 (Fig. 1e), which binds to a surface unaffected by the mutations (Fig. 1a, b). Antibody 83-7 demonstrated cross-reactivity with endogenous CHO INSR, as evidenced by positive staining of CHO Flp-In parent cells, while the other antibodies did not detectably cross-react.
Assessment of mutant INSR autophosphorylation in response to antibody and/or insulin
Trans-autophosphorylation of tyrosines in the intracellular INSR is the first detectable signalling event after insulin binding, so the ability of insulin and antibodies to induce tyrosine phosphorylation of mutant INSR was next examined using anti-myc immunoprecipitation and europium-based immunoassay. Most mutant receptors (P193L, F248C, R252C, S323L, F382V, D707A, P1178L) demonstrated diminished maximal autophosphorylation response to insulin, ranging from 0 to 27% WT (Fig. 2a–j, Table 2, data not shown for non-responsive P1178L). However, R118C, I119M and K460E showed autophosphorylation comparable with WT, and so were not studied further. Altered insulin EC50 was discernible only for S323L (Table 2), although the insulin concentration range tested and the small magnitude of responses precluded precise determinations.
Insulin- and antibody-stimulated autophosphorylation of WT and mutant INSR. CHO Flp-In cells stably expressing either human WT or mutant INSR (as indicated) were serum starved prior to 10 min stimulation with increasing concentrations of insulin, antibody (83-7, 83-14) or control IgG (black lines), or increasing concentrations of insulin in the presence of 10 nmol/l antibody (grey lines). Cells were lysed and myc-tagged receptors were immunocaptured on 96 well plates and then incubated with biotin-conjugated 4G10 platinum antibody to detect phosphorylated tyrosine residues. Europium-labelled streptavidin was used to detect bound anti-phosphotyrosine antibody 4G10 by time-resolved fluorescence. The data points are the mean ± SEM of duplicate samples from three independent experiments and plotted on a log10 scale for the x-axis. Error bars are shown when larger than size of the symbols. In (a–j), single treatments are shown as follows: black circles (solid line), insulin; black up-pointing triangle (dashed line), 83-7; black down-pointing triangle (dotted line), 83-14; black open circles (solid line), control IgG. In (k–t), dual treatments are denoted by: grey up-pointing triangle (dashed line), insulin +10 nmol/l 83-7; grey down-pointing triangle (dotted line), insulin +10 nmol/l 83-14; grey circles (dotted/dashed line), insulin +10 nmol/l control IgG. pEC50 values are presented in Table 2
Antibodies 83-7 and 83-14 alone also elicited autophosphorylation of WT and all mutant INSRs except F248C and P1178L. In most instances, antibody response was lower than insulin response (Fig. 2a–j, Table 2); however, for S323L the maximal autophosphorylation response to 83-7 and 83-14 was similar to that with insulin (Fig. 2g), while D707A was activated by antibodies but not insulin (Fig. 2j).
We next evaluated responses to insulin +10 nmol/l antibody, based on evidence that this concentration elicits the maximal response [18, 20]. In the presence of antibodies 83-7 and 83-14, the maximal response of WT and mutant INSRs to insulin was increased without affecting potency (although EC50 values were not precisely determined) (Fig. 2k–t, Table 2). This was observed across all mutant receptors except the kinase-dead P1178L [24, 25]. Antibodies 18-44 and 18-146 elicited smaller effects than 83-7 and 83-14, and for clarity of presentation data for these antibodies are shown in the ESM (ESM Results, ESM Figs 1, 2, ESM Table 7).
Generation of a novel adipocyte cell model of insulin receptoropathy
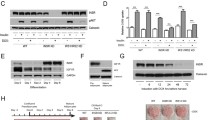
To assess antibody-induced signalling downstream from the INSR, an adipocyte model of insulin receptoropathy was generated. A tetracycline (tet)-responsive microRNA (miR)-short hairpin (sh)RNA selectively targeting murine Insr was transduced into 3T3-L1 pre-adipocytes to generate a stable clone (Fig. 3a). This was transduced with lentiviruses encoding C-terminal myc-tagged WT or mutant hINSR, also controlled by tet-responsive elements (Fig. 3b), generating cells in which DOX simultaneously knocked down endogenous murine Insr and induced overexpression of myc-tagged human INSR. This system permitted pre-adipocyte differentiation uncompromised by mutant receptor expression before induction of Insr knockdown/hINSR re-expression in adipocytes (Fig. 3c, d). The DOX concentration producing maximal Insr knockdown resulted in overexpression of hINSR transgenes (Fig. 3c, e); however, the receptor-processing defects observed in CHO cells (Fig. 1c) were preserved. The C-terminal myc-tag enabled discrimination of endogenous mouse and ectopic human INSR by size shift of the INSR β subunit on immunoblotting, or by anti-myc antibodies (Fig. 3e). The pre-adipocyte cell lines generated differentiated efficiently into mature adipocytes, as evidenced by Oil Red O staining (Fig. 3f).
Generation of a novel stable 3T3-L1 adipocyte model of insulin receptoropathy. (a) Concatenated miR-shRNAs targeting murine Insr in exon 2 and exon 9 preceded by GFP under the control of a tet-responsive element was packaged into third-generation lentivirus to enable transduction of 3T3-L1 pre-adipocytes. The exploded view shows the nucleotide mismatches between the mouse Insr targeted by each miR-shRNA with the human INSR sequence. Green shaded elements of the transgene are inducible by the addition of DOX. Transduced 3T3-L1 pre-adipocytes underwent single cell clonal selection in the presence of hygromycin to generate 3T3-L1 MmINSRKD. (b) 3T3-L1 MmINSRKD cells were then transduced with a second lentivirus encoding C-terminal myc-tagged human INSR transgenes under the control of a tet-responsive element and underwent polyclonal selection in the presence of neomycin to generate 3T3-L1 MmINSRKD hINSR. (c) Western blots of whole-cell lysates from day 10 mature 3T3-L1 MmINSRKD and 3T3-L1 MmINSRKD hINSR WT cells grown in the presence of increasing concentrations of DOX for 72 h. (d) Densitometry analysis of western blots from three independent experiments demonstrating knockdown of endogenous mouse Insr and expression of human INSR with increasing concentrations of DOX. (e) Western blots of whole-cell lysates from day 16 mature 3T3-L1 MmINSRKD and 3T3-L1 MmINSRKD hINSR (mutant INSR as indicated) cells grown in the presence of 1 μg/ml DOX for 10 days. (f) Oil Red O staining of lipid accumulation in day 10 mature 3T3-L1 MmINSRKD and 3T3-L1 MmINSRKD hINSR WT or mutant (as indicated) cells grown ± DOX (1 μg/ml) for 72 h. GFP, green fluorescent protein; Hygro, hygromycin resistance; IRES, internal ribosome entry site; Mm, murine; Neo, neomycin resistance; rtTA3, reverse tetracycline-controlled transactivator; TRE, tet-response element; Ubi-C, ubiquitin C promoter
Activation of signalling downstream from mutant INSRs by insulin and antibody
Plasma insulin concentration in human insulin receptoropathies lies between 0.3 and 3 nmol/l in the fasting state (ESM Table 6), and at least an order of magnitude higher when fed. We used an insulin concentration of 10 nmol/l, mimicking the fed disease state. WT INSR autophosphorylation was strongly induced by insulin (Fig. 4a, b), but was undetectable after receptor knockdown alone (ESM Fig. 2c, d). Otherwise, the pattern of autophosphorylation of overexpressed receptors in response to insulin and/or antibody was similar to that seen in CHO cells. Thus, antibodies 83-7 and 83-14 alone induced WT receptor autophosphorylation on Y1162/Y1163, while antibodies 18-44 and 18-146 were less effective (ESM Fig. 2, ESM Results). Insulin-stimulated autophosphorylation was reduced by 75-100% in mutant INSRs compared with WT. Although antibodies alone induced low-level phosphorylation of mutant INSRs (<10%), the responses of S323L and D707A to antibodies 83-7 and 83-14 were equal to or greater than those to insulin (Fig. 4g–j). Combined insulin and antibody treatment enhanced phosphorylation of each mutant INSR in the case of 83-7 and 83-14, likely synergistically (Fig. 4, ESM Fig. 2).
Activation of signalling pathways downstream of WT and mutant INSR by insulin and antibody stimulation. 3T3-L1 MmINSRKD hINSR WT (a, b), P193L (c, d), R252C (e, f), S323L (g, h), D707A (i, j) and P1178L (k, l) adipocytes were grown in the presence of 1 μg/ml DOX for 8 days prior to overnight serum starvation on day 13 of differentiation. Adipocytes were then stimulated with either 10 nmol/l insulin (red bars), 10 nmol/l antibody (83-7, 83-14 or control IgG; dark grey bars) or 10 nmol/l insulin containing 10 nmol/l antibody (light grey bars) for 10 min at 37°C/5% CO2. Following stimulation, cells were washed and snap frozen prior to lysis and western blot. Bar graphs show p-INSRβ, p-ERK1/2, p-Akt, p-GSK3α, p-p70S6K and p-AS160 densitometry after normalisation for each sample by the sum aggregate of multiple proteins (total INSRβ, myc-tagged INSRβ, ERK1/2, Akt, GSK3α/β, p70S6K and calnexin) for each biological replicate. Data are the mean ± SD of three independent experiments and are expressed relative to hINSR WT response to insulin stimulation; individual data points are shown in scatter plots. For clarity of presentation, only key data are presented here; an extended version appears as ESM Fig. 2
Akt2/PKBβ transduces metabolic actions of insulin after phosphorylation of T308 and S473. p-Akt2 phosphorylates substrates including glycogen synthase kinase (GSK3α/β), which regulates glycogen synthesis, p70 S6 kinase (p70S6K), which stimulates protein synthesis, and AS160, which encodes a GTPase-activating protein that restrains GLUT4 vesicle translocation until phosphorylated. Insulin treatment of WT INSR induced strong Akt phosphorylation at both sites (Fig. 4a, b), and this was severely attenuated by knockdown of endogenous Insr or by knockdown with re-expression of the kinase-dead P1178L mutant (Fig. 4k, l). Attenuation of signalling was also apparent downstream of Akt, with phosphorylation of p70S6K and GSK3 only modestly impaired, and AS160 phosphorylation unaffected.
Several patterns were seen across the panel of mutants studied. In D707A receptor-expressing cells (Fig. 4i, j), insulin-induced phosphorylation of Akt and its substrates was severely attenuated, while a progressive ‘escape’ from signalling impairment was seen in mutants S323L (Fig. 4g, h), F248C (ESM Fig. 2g, h) and F382V (ESM Fig. 2m, n), with lesser impairment of Akt phosphorylation than of receptor autophosphorylation, and only partial inhibition at downstream substrates. P193L (Fig. 4c, d) and R252C (Fig. 4e, f) demonstrated similar insulin-induced Akt and Akt substrate phosphorylation to WT receptor.
Antibodies alone stimulated Akt and Akt substrate phosphorylation in all cells except those overexpressing the kinase-dead P1178L mutant (Fig. 4k, l). For S323L and D707A mutants, antibodies 83-7 and 83-14 stimulated greater phosphorylation than insulin alone, by virtue of the low response of those mutants to insulin (Fig. 4g–j). Co-treatment of cells with insulin and antibodies 83-7 and 83-14 enhanced Akt and Akt substrate phosphorylation with respect to insulin alone, without evidence of synergy. The additive effects of insulin and antibody co-stimulation were generally less than those observed for receptor autophosphorylation in CHO cells (Fig. 2k–t).
Activation of the INSR by insulin stimulates not only phosphoinositide 3-kinase (PI3K)/Akt, but also RAS/RAF/mitogen-activated protein kinase kinase (MEK)/ERK signalling, through both IRS-dependent and IRS-independent mechanisms [31]. Activation of this pathway is a surrogate for mitogenicity of insulin analogues [32], which is important in view of concerns about long-term cancer risks of analogues with pro-proliferative activity. Insulin treatment of WT INSR induced robust phosphorylation of ERK1/2 at Y204/Y187, with each mutant INSR displaying reduced phosphorylation in response to insulin compared with WT (Fig. 4). Antibody treatment of mutant or WT INSR did not induce ERK1/2 phosphorylation, while dual stimulation with antibody + insulin did not increase ERK1/2 phosphorylation compared with insulin alone. Higher basal ERK1/2 phosphorylation was observed in cells with Insr knockdown alone (ESM Fig. 2c, d), or with Insr knockdown and P1178L receptor overexpression (Fig. 4k, l), but this did not change with any treatment.
Effect of insulin and/or antibody on glucose uptake
Glucose uptake is a key outcome of INSR activation and was assessed in the 3T3-L1 model. Parent 3T3-L1 cells and cells harbouring the Insr-knockdown construct but not treated with DOX displayed similar high levels of insulin-stimulated glucose uptake (ESM Fig. 3a, b), but insulin did not stimulate uptake in conditional Insr-knockdown cells treated with doxycycline (Fig. 5i). Cells with endogenous mouse Insr knockdown and WT hINSR re-expression, in contrast, demonstrated only a 1.8-fold increase in glucose uptake on insulin stimulation (ESM Fig. 3a). The apparently poor response to insulin was due to increased basal glucose uptake in WT receptor-overexpressing cells (ESM Fig. 3b). Basal uptake among mutant receptor-expressing cell lines reflected mutant receptor function (ESM Fig. 3c).
Insulin- and antibody-stimulated glucose uptake via WT and mutant INSR. 3T3-L1 MmINSRKD hINSR WT (a), P193L (b), F248C (c), R252C (d), S323L (e), F382V (f), D707A (g), P1178L (h) and MmINSRKD (i) adipocytes were grown in the presence of 1 μg/ml DOX for 10 days prior to overnight serum starvation on day 15 of differentiation. The cells were stimulated for 30 min with either 10 nmol/l insulin (red bars), 10 nmol/l antibody (83-7, 83-14 or control IgG; dark grey bars) or 10 nmol/l insulin containing 10 nmol/l antibody (light grey bars) prior to the addition of 2-deoxy-d-glucose for 5 min. Cells were then washed, lysed and assessed for 2-deoxy-d-glucose uptake. Bar chart data are the mean ± SD from three independent experiments; scatter plots indicate the mean of triplicates from each independent experiment. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparison test: *p < 0.05, **p < 0.01 and ***p < 0.001, vs unstimulated basal; †p < 0.05, ††p < 0.01 and †††p < 0.001, vs 10 nmol/l insulin treatment; ‡p < 0.05, ‡‡p < 0.01 and ‡‡‡p < 0.001, vs 10 nmol/l IgG control treatment; §p < 0.05, §§p < 0.01 and §§§p < 0.001, vs 10 nmol/l insulin in the presence of 10 nmol/l IgG control. 2-DG, 2-deoxy-d-glucose
Despite reduced dynamic range in the assay, insulin stimulated glucose uptake via WT, P193L, F248C, R252C and F382V receptors (Fig. 5a, b, d, f, respectively). Insulin-stimulated uptake was similar in cells expressing P193L, R252C or F382V receptor and those expressing WT receptor, but was reduced in cells expressing the F248C mutant. No stimulation of glucose uptake was seen in cells expressing S323L, D707A or P1178L receptors (Fig. 5e, g, h, respectively).
Antibodies 83-7 and 83-14 alone stimulated glucose uptake via P193L, S323L, F382V and D707A receptors (Fig. 5), while antibodies 18-44 and 18-146 were again less effective across the full range of mutants (ESM Fig. 4). While the magnitude of antibody-stimulated uptake was less than that seen with insulin via WT, P193L, F248C, R252C and F382V receptors, antibodies 83-7, 83-14 and 18-44 were more effective than insulin at stimulating glucose uptake via D707A. Dual treatment with antibodies + insulin did not enhance glucose uptake compared with insulin alone acting via WT, P193L, F248C, R252C and F382V receptors, or antibody alone when acting via S323L and D707A receptors.
Discussion
Recessive insulin receptoropathies feature failure to thrive, extreme metabolic derangement, childhood mortality and poor response to therapy. Longitudinal studies suggest a steep relationship between residual INSR function and clinical outcome: loss of 50% INSR function, as in the parents of infants with Donohue syndrome, does not produce insulin resistance in lean people. Heterozygous dominant negative mutations produce severe insulin resistance, diagnosed peripubertally in girls and later in men, and reduce receptor function to 25% or less of WT. The severe recessive receptoropathies that this study focuses on confer greater loss of function. However, even with 0–25% residual function, a range of phenotypes is seen, with complete loss of function producing Donohue syndrome and lethality in infancy, but less extreme loss of function producing Rabson–Mendenhall syndrome, with survival to the second or third decade. These observations suggest that even modest improvements in receptor signalling in recessive disease may have decisive clinical benefit.
Many pathogenic INSR mutations are known, including more than 100 missense mutations. A subset are expressed at the cell surface, but show impaired insulin binding, signal transduction or internalisation and recycling. This subset may be amenable to non-conventional activation by antibody. Proof of this principle came from demonstration that two bivalent antibodies stimulated kinase activity of a single solubilised mutant receptor (F382V [7]), and, independently, that one bivalent antibody increased glycogen synthesis acting via a mutant receptor expressed in intact cells (S232L [8]). We extend these findings with systematic characterisation of multiple receptor mutants and antibodies in two cellular systems, assaying physiologically important responses including adipocyte glucose uptake.
One of the mutants assessed, F248C, is novel. It lies close to the R252C mutant, which is expressed but exhibits impaired internalisation after insulin exposure [33]. F248C shows minor reduction in cell surface expression, but insulin-stimulated receptor autophosphorylation and downstream signalling are severely impaired. Across known mutants, our data generally agree with prior studies. Assay of receptor autophosphorylation in CHO cells using immunocapture of myc-tagged receptor prior to immunoassay demonstrated signalling defects more clearly than phosphotyrosine immunoblotting in the 3T3-L1 overexpression system, likely reflecting the inherently greater dynamic range of immunoassay allied to use of a generic anti-phosphotyrosine antibody.
We confirmed that S323L and F382V receptors can be activated by antibodies and extended these observations to a wider range of mutants. Previous studies suggest that receptor activation by antibody depends on receptor cross-linking rather than reaction at specific epitopes [19]. Consistent with this, two of the antibodies we employed, 83-7 and 83-14, are both effective despite recognising different epitopes and having different effects on insulin binding. Antibodies 18-44 and 18-146 consistently elicited much smaller responses, although 18-44 has previously been found to exert insulin-like activity on primary human adipocytes [20]. Differences among antibodies are likely to reflect differences of affinity and/or steric constraints on cross-linking receptors.
The mutants showing the largest antibody response were S323L and D707A, both being activated by antibodies similarly to WT receptor, and to a greater extent than by insulin. Such mutants with ‘pure’ insulin-binding defects are particularly attractive therapeutic targets. Other mutants studied in both cell systems (P193L, F248C, R252C and F382V) showed some activation of Akt, GSK3, AS160 and glucose uptake by antibodies. In these cases, responses were less than for WT receptor or those induced by insulin. Testing the therapeutic potential of antibodies against such mutants is warranted in vivo, where antibody signalling may be prolonged compared with insulin signalling because of slower receptor internalisation. Indeed, a previously studied anti-INSR antibody showed markedly greater hypoglycaemic effects in vivo in WT animals than had been apparent in cell culture models [13].
Antibodies would be a particularly appealing therapeutic proposition were they to exhibit synergy with insulin in receptor stimulation, amplifying insulin action rather than simply imposing a tonic signal. The current studies have not addressed this in detail, although suggestive evidence for synergic stimulation of WT receptor and some mutant receptors is seen. This was not mirrored by detectable synergistic activation of downstream signalling or metabolic endpoints, possibly because maximal downstream signalling requires only submaximal receptor autophosphorylation. It remains possible that insulin–antibody synergy does exist but was obscured under the conditions of the experiments undertaken, which pragmatically employed relatively high concentrations of insulin and antibody.
Early cellular studies of antibody-induced INSR activation were interpreted as suggesting that antibodies elicit greater downstream responses than expected from low levels of receptor autophosphorylation [16, 34,35,36]. These observations were later argued to have a methodological basis, hinging on lower sensitivity in detecting tyrosine phosphorylation than downstream signalling [37, 38]. This is, in part, because signal amplification is an inherent property of signal transduction cascades. Our observation of apparent ‘escape’ from signalling inhibition in the face of efficient Insr knockdown in 3T3-L1 adipocytes supports this contention, as activation of residual receptors is undetectable directly but is observable downstream, owing to signal amplification.
Importantly, receptor activation by antibodies leads to selective Akt phosphorylation, which is critical for metabolic actions of insulin, with little or no ERK phosphorylation. As activation of the RAS/RAF/MEK/ERK pathway is mitogenic, this is an encouraging property of antibodies for translational purposes, suggesting that they may exert metabolic benefits without undue mitogenic activity. Similar dissociation between activation of Akt and ERK has also been observed following INSR activation by the peptide ligand S597 [39] and in previous studies with anti-receptor antibodies [40]. The mechanism underlying such biased agonism is poorly understood, although IRS proteins may be preferentially phosphorylated by plasma membrane-associated receptor [33, 41], whereas receptor internalisation is required for full ERK activation [33, 42].
We studied only a limited number of insulin and antibody concentrations. While these were selected with reference to prior studies and observed blood insulin concentrations in insulin receptoropathy, the conditions we describe may not be most relevant in vivo, where insulin and antibody concentrations in the interstitial space of target tissues may be variable and different. Moreover, receptor overexpression may have partially overcome receptor dysfunction and made beneficial effects of antibody more difficult to observe. Finally, in the paradigm of acute antibody stimulation with static signalling endpoints, issues such as the potential of long-term antibody treatment to downregulate receptors, and the effect of antibodies on receptor recycling kinetics in vivo have not been addressed. This is likely to be particularly important for the subset of mutants (e.g. I119M, K460E) where acute insulin stimulations studies are normal, as in this and other reports, but which confer extreme insulin resistance in vivo.
Conclusions
Multiple monoclonal antibodies can bind and activate mutated cell surface INSR to a potentially clinically significant degree. Experience in WT animals [13] and theoretical considerations argue that effects of anti-INSR antibodies in vivo may be greater than in cells, so further studies in animal models are warranted.
Data availability
All data generated or analysed during this study are included in this published article and the ESM.
Abbreviations
- 3T3-L1 MmINSRKD:
-
Murine Insr-knockdown cells
- AS160:
-
Akt substrate of 160 kDa
- CHO:
-
Chinese hamster ovary
- DOX:
-
Doxycycline
- ERK1/2:
-
Extracellular signal-regulated kinase 1/2
- GSK3:
-
Glycogen synthesis kinase 3
- INSR:
-
Insulin receptor
- MEK:
-
Mitogen-activated protein kinase kinase
- MOI:
-
Multiplicity of infection
- p70S6K:
-
Ribosomal protein S6 kinase β-1
- shRNA:
-
Short hairpin RNA
- tet:
-
Tetracycline
- TIFF:
-
Tag image file format
- WT:
-
wild-type
References
Ebina Y, Ellis L, Jarnagin K et al (1985) The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell 40:747–758
Ullrich A, Bell R, Chen EY et al (1985) Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature 313:756–761
Yoshimasa Y, Seino S, Whittaker J et al (1988) Insulin-resistant diabetes due to a point mutation that prevents insulin proreceptor processing. Science 240:784–787
Kadowaki T, Bevins CL, Cama A et al (1988) Two mutant alleles of the insulin receptor gene in a patient with extreme insulin resistance. Science 240:787–790
Semple RK, Savage DB, Brierley GV, O’Rahilly S (2016) Syndromes of severe insulin resistance and/or lipodystrophy. In: Weiss RE, Refetoff S (eds) Genetic diagnosis of endocrine disorders, 2nd edn. Academic Press, Cambridge, MA, pp 307–324
Taylor SI, Cama A, Accili D et al (1992) Mutations in the insulin receptor gene. Endocr Rev 13:566–595
Lebrun C, Baron V, Kaliman P et al (1993) Antibodies to the extracellular receptor domain restore the hormone-insensitive kinase and conformation of the mutant insulin receptor valine 382. J Biol Chem 268:11272–11277
Krook A, Soos M, Kumar S, Siddle K, O’Rahilly S (1996) Functional activation of mutant human insulin receptor by monoclonal antibody. Lancet 347:1586–1590
Ayyar BV, Arora S, O’Kennedy R (2016) Coming-of-age of antibodies in cancer therapeutics. Trends Pharmacol Sci 37:1009–1028
Reichert JM (2016) Antibodies to watch in 2017. MAbs 1–61
Johnson KW, Neale A, Gordon A et al (2017) Attenuation of insulin action by an allosteric insulin receptor antibody in healthy volunteers. J Clin Endocrinol Metab 102:3021–3028
Bedinger DH, Kieffer DA, Goldfine ID, Roell MK, Adams SH (2015) Acute treatment with XMetA activates hepatic insulin receptors and lowers blood glucose in normal mice. J Cell Biochem 116:2009–2019
Bhaskar V, Lau A, Goldfine ID et al (2013) XMetA, an allosteric monoclonal antibody to the insulin receptor, improves glycaemic control in mice with diet-induced obesity. Diabetes Obes Metab 15:272–275
Bhaskar V, Goldfine ID, Bedinger DH et al (2012) A fully human, allosteric monoclonal antibody that activates the insulin receptor and improves glycemic control. Diabetes 61:1263–1271
Bezwada P, Zhao J, Der K et al (2016) A novel allosteric insulin receptor-activating antibody reduces hyperglycaemia without hypoglycaemia in diabetic cynomolgus monkeys. J Pharmacol Exp Ther 356:466–473
Soos MA, O’Brien RM, Brindle NP et al (1989) Monoclonal antibodies to the insulin receptor mimic metabolic effects of insulin but do not stimulate receptor autophosphorylation in transfected NIH 3T3 fibroblasts. Proc Natl Acad Sci U S A 86:5217–5221
Soos MA, Siddle K, Baron MD et al (1986) Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J 235:199–208
Siddle K, Soos MA, O’Brien RM, Ganderton RH, Taylor R (1987) Monoclonal antibodies as probes of the structure and function of insulin receptors. Biochem Soc Trans 15:47–51
O’Brien RM, Soos MA, Siddle K (1987) Monoclonal antibodies to the insulin receptor stimulate the intrinsic tyrosine kinase activity by cross-linking receptor molecules. EMBO J 6:4003–4010
Taylor R, Soos MA, Wells A, Argyraki M, Siddle K (1987) Insulin-like and insulin-inhibitory effects of monoclonal antibodies for different epitopes on the human insulin receptor. Biochem J 242:123–129
Shin KJ, Wall EA, Zavzavadjian JR et al (2006) A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc Natl Acad Sci U S A 103:13759–13764
Miki H, Yamauchi T, Suzuki RYO et al (2001) Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol Cell Biol 21:2521–2532
Yamamoto N, Ueda M, Sato T et al (2011) Measurement of glucose uptake in cultured cells. Curr Protoc Pharmacol 1–22
Krook A, Moller DE, Dib K, O’Rahilly S (1996) Two naturally occurring mutant insulin receptors phosphorylate insulin receptor substrate-1 (IRS-1) but fail to mediate the biological effects of insulin. Evidence that IRS-1 phosphorylation is not sufficient for normal insulin action. J Biol Chem 271:7134–7140
Krook A, Whitehead JP, Dobson SP et al (1997) Two naturally occurring insulin receptor tyrosine kinase domain mutants provide evidence that phosphoinositide 3-kinase activation alone is not sufficient for the mediation of insulin’s metabolic and mitogenic effects. J Biol Chem 272:30208–30214
Rouard M, Bass J, Grigorescu F et al (1999) Congenital insulin resistance associated with a conformational alteration in a conserved β-sheet in the insulin receptor L1 domain. J Biol Chem 274:18487–18491
Croll TI, Smith BJ, Margetts MB et al. (2016) Higher-resolution structure of the human insulin receptor ectodomain: multi-modal inclusion of the insert domain. Struct Des 1–8
Menting JG, Whittaker J, Margetts MB et al (2013) How insulin engages its primary binding site on the insulin receptor. Nature 493:241–245
Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R (2009) Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30:586–623
Rouard M, Macari F, Bouix O et al (1997) Identification of two novel insulin receptor mutations, Asp59Gly and Leu62Pro, in type A syndrome of extreme insulin resistance. Biochem Biophys Res Commun 234:764–768
Siddle K (2012) Molecular basis of signalling specificity of insulin and IGF receptors: neglected corners and recent advances. Front Endocrinol 3:1–24
Sciacca L, Cassarino MF, Genua M, Pandini G (2010) Insulin analogues differently activate insulin receptor isoforms and post-receptor signalling. Diabetologia 53:1743–1753
Hamer I, Foti M, Emkey R et al (2002) An arginine to cysteine 252 mutation in insulin receptors from a patient with severe insulin resistance inhibits receptor internalisation but preserves signalling events. Diabetologia 45:657–667
Zick Y, Rees-Jones RW, Taylor SI, Gorden P, Roth J (1984) The role of antireceptor antibodies in stimulating phosphorylation of the insulin receptor. J Biol Chem 259:4396–4400
Sung CK, Maddux BA, Hawley DM, Goldfine ID (1989) Monoclonal antibodies mimic insulin activation of ribosomal protein S6 kinase without activation of insulin receptor tyrosine kinase. Studies in cells transfected with normal and mutant human insulin receptors. J Biol Chem 264:18951–18959
Forsayeth JR, Caro JF, Sinha MK, Maddux BA, Goldfine ID (1987) Monoclonal antibodies to the human insulin receptor that activate glucose transport but not insulin receptor kinase activity. Proc Natl Acad Sci U S A 84:3448–3451
Brindle NP, Tavare JM, Dickens M, Whittaker J, Siddle K (1990) Anti-insulin receptor monoclonal antibody-stimulated tyrosine phosphorylation in cells transfected with human insulin receptor cDNA. Biochem J 268:615–620
Steele-Perkins G, Roth R (1990) Insulin-mimetic anti-insulin receptor monoclonal antibodies stimulate receptor kinase activity in intact cells. J Biol Chem 265:9458–9463
Jensen M, Hansen B, De Meyts P, Schäffer L, Ursø B (2007) Activation of the insulin receptor by insulin and a synthetic peptide leads to divergent metabolic and mitogenic signalling and responses. J Biol Chem 282:35179–35186
Bedinger DH, Goldfine ID, Corbin JA, Roell MK, Adams SH (2015) Differential pathway coupling of the activated insulin receptor drives signalling selectivity by XMetA, an allosteric partial agonist antibody. J Pharmacol Exp Ther 353:35–43
Rakatzi I, Ramrath S, Ledwig D et al (2003) A novel insulin analogue with unique properties: LysB3, Glu B29 insulin induces prominent activation of insulin receptor substrate 2, but marginal phosphorylation of insulin receptor substrate 1. Diabetes 52:2227–2238
Ceresa BP, Kao AW, Santeler SR, Pessin JE (1998) Inhibition of clathrin-mediated endocytosis selectively attenuates specific insulin receptor signal transduction pathways. Mol Cell Biol 18:3862–3870
Acknowledgements
We thank C. Gewert and D. Newby (Institute of Metabolic Science, University of Cambridge, Cambridge, UK) for technical support. Some of the data were presented as abstracts at the Diabetes UK Professional Conference in London, UK, 11–13 March 2015 and Manchester, UK, 7–10 March 2017, and the International Symposium on Insulin Receptor and Insulin Action 2017, Nice, France, 20–22 April 2017.
Funding
Funding was from an Open Funding grant from the Diabetes Research and Wellness Foundation (to GVB), and a project grant from Diabetes UK (to RKS). RKS is funded by the Wellcome Trust (WT098498), and core support was provided by the Medical Research Council [MRC_MC_UU_12012/5] and the UK National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
All authors contributed to experimental design, data acquisition and analysis, and writing the manuscript. All authors approved the final version. RKS is the guarantor of this work.
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Electronic supplementary material
ESM
(PDF 9825 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brierley, G.V., Siddle, K. & Semple, R.K. Evaluation of anti-insulin receptor antibodies as potential novel therapies for human insulin receptoropathy using cell culture models. Diabetologia 61, 1662–1675 (2018). https://doi.org/10.1007/s00125-018-4606-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4606-2