Abstract
Here, we describe the surgical technique for implanting a new, active, transcutaneous bone conduction hearing aid. The implant technology is based on a system that has been in use reliably since 2012. The geometry of the new implant has been adapted based on experience with previously introduced implants. The surgery was feasible, standardized, and safe. Due to the optimized geometric design that improved the bone fit, it is not necessary to use specialized, detailed preoperative planning, except in challenging anatomical conditions; e.g., in young children, malformations, poor pneumatization, or after a canal wall down mastoidectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bone conduction hearing implants
Bone conduction hearing implants improve hearing in patients with conductive or mixed hearing loss, who do not experience hearing improvement with conventional hearing aids or conventional middle ear surgery (tympanoplasty, creation of an ear canal in atresia) [4, 8]. However, it is necessary to consider the limitations of these systems with respect to their audiological indication criteria [19]. In patients with single-sided deafness, bone conduction hearing implants can be used to rehabilitate hearing through “contralateral routing of signal” (CROS) [4, 23].
In the indications described above, bone conduction hearing implants have successfully restored hearing for decades with percutaneous mechanical energy transfer. However, the disadvantage of these implants is that they penetrate the skin, which poses the inherent risks of skin reactions and infections. Fussey et al. analyzed long-term effects of percutaneous bone conduction hearing implants in children. They reported that 77% of children experienced soft tissue complications that required treatment [10]. When soft tissue infections occur, the sound processor cannot be worn due to interference from the local treatment. This situation places constraints on hearing rehabilitation for the patient. A previous meta-analysis showed that these complications led to implant loss in 1.6–17.4% of patients [11].
With the introduction of minimally invasive implantation techniques and the avoidance of skin thinning with longer abutments, the complication rate appears to have improved. However, long-term follow-up studies in larger cohorts are lacking [24]. The typical soft tissue complications associated with percutaneous bone conduction hearing implants can be avoided with transcutaneous systems. With these systems, the active (vibrating) component is implanted subcutaneously, which avoids a percutaneous anchor. Additionally, the risk of skin irritation is reduced with inductive energy transfer, which avoids permanent pressure. One of these systems is the transcutaneous bone conduction implant (BCI) called “Bonebridge” (MED-EL, Innsbruck, Austria). The first generation (BCI 601) was introduced in 2012. In 2014, the BCI 601 was also approved for use in children, starting from age 5 years [3, 35].
The audiological outcome showed results similar to those reported for percutaneous bone conduction hearing implants [18]. However, the complication rate and the rate of revision surgery were lower with the transcutaneous system than with percutaneous implants [11, 18, 26]. These features have given rise to a trend towards using bone conduction hearing aid implants with transcutaneous inductive energy transfer, rather than percutaneous hearing aid implants [31].
The active (vibrating) component of the BCI 601 is responsible for energy transfer to the skull and, thus, to the inner ear. This component is called the bone conduction-floating mass transducer (BC-FMT). Based on three-dimensional (3D) reconstructions of temporal bones and “virtual surgery,” the BC-FMT can be adequately fitted to the mastoid bone in 77% of women and 81% of men, but less than 50% of children under 8 years of age [20]. Therefore, it was necessary to develop smaller active transcutaneous bone conduction hearing implants.
Further development of the BCI 601 (the BCI 602) reduced the penetration depth of the BC-FMT from 8.7 to 4.5 mm. This was achieved by increasing the BC-FMT diameter from 15.8 to 18.2 mm and locating some components of the BC-FMT above the bony surface. With the additional use of 1‑mm spacing washers (BCI 602 Lifts), the implantation depth can be further reduced to 3.5 mm. This configuration elevates the BC-FMT to 5.1 mm above the bony surface. The anchor holes in the fixation wings are 24.4 mm apart (BCI 601: 23.8 mm; Fig. 1).
Comparison of the bone conduction implants (BCI) 601 and 602. Images show the new active bone conduction hearing system, BCI 602 (right), and the previous model, BCI 601 (left), (© MED-EL, Innsbruck, Austria, with permission). Virtual three-dimensional (3D) models of the bone conduction-floating mass transducers (BC-FMT) are used for preoperative planning. All measurements are expressed in millimeters
This article describes the surgical technique for implanting the new active transcutaneous bone conduction hearing implant, BCI 602 (MED-EL, Innsbruck, Austria) with optimized geometry.
Surgical technique
The implantable component of the bone conduction implant BCI 602 consists of an FMT with the electronics and an attachment magnet surrounded by the receiver coil (Fig. 1). The external part, the audio processor, is held in place by the magnet.
Preoperatively, the temporal bone is evaluated to determine placement of the BC-FMT. Several methods and tools have been described for this task, varying from a detailed visual analysis and two-dimensional (2D) measurements of standard computed tomography (CT) scans to sophisticated planning tools and preoperative “virtual surgery” [2, 6, 7, 12, 13, 15, 17, 27,28,29,30, 34].
Surgery can be performed with either local or general anesthesia. All surgeries described here were performed with the patient under general anesthesia and additional periauricular infiltration with Ultracain/Suprarenin (articaine/adrenaline). The optimal position of the BC-FMT was then transferred to the surgical field (Fig. 2).
Landmarks for transferring the preoperatively determined implant position into the surgical field. Suitable landmarks include the mastoid tip, the anterior wall of the external ear canal or its remnants, the lateral orbital rim (not shown here), or the temporomandibular joint [17]. During planning, bone thickness is measured in the area where the self-drilling cortical screws will be placed to fix the bone conduction-floating mass transducer [33]
Surgery can be performed with either local or general anesthesia
The surgeon should consider a number of precautions. First, the receiver coil and the audio processor should not come into contact with scars from previous surgeries. Pressure due to contact with the magnets could lead to impaired capillary perfusion and, in the worst case scenario, necrosis in the overlying scarred skin. Skin and pericranial incisions should not be superimposed. In cases where an aesthetic auricular reconstruction is planned at a later stage due to atresia, or to leave room for this possibility, a “posterior atresia incision” can be performed, as described by Frenzel et al. (2010). In this procedure, an incision cuts through all layers, following which a subperiosteal dissection is performed. Thus, the physical integrity of the tissue layers around the ear remnant are preserved (Fig. 3; [9]).
Planning the incisions. The skin and pericranial incisions should not be superimposed. Exceptions include cases of atresia, with a possible aesthetic auricular reconstruction planned at a later stage. In these cases, the position of the ear is marked according to standard recommendations. The posterior incision is made through all layers at a distance of approximately 15 mm from the later helix [9]
Next, only the implant bed for the BC-MFT is drilled. Despite careful preoperative planning, the implant bed might reach the dura, the sigmoid sinus, an ear canal remnant, or an open mastoid cavity. Therefore, the authors typically start by drilling the implant bed in the center of the BC-FMT position to a depth of 4.5 mm with a small-diameter drill bit, approximately 5 mm in diameter. The implant bed is then gradually enlarged. By successively moving the drill bit away from the structures mentioned, they can be avoided (Fig. 4A). The adequate size and shape of the implant bed can be evaluated with a sterile “BCI 602 Sizer Kit” (Fig. 4B). Alternatively, an adapted sterile ruler and an intraoperatively “created” depth gauge (the authors use a 0.7-mm suction tip, off-label) have proven useful for this measurement (Fig. 4C, D).
Creating the implant bed. A Drilling of the implant bed is started in the center of the position planned for the bone conduction-floating mass transducer. The depth is 4.5 mm and the diameter is relatively small, approximately 5 mm. The implant bed is successively enlarged by moving the drill bit away from critical structures (sigmoid sinus, dura, ear canal, open mastoid cavity) to avoid damage. B The adequacy of the size and shape of the implant bed can be evaluated with a sterile “BCI 602 Sizer Kit.” Alternatively, the implant bed can be measured with a sterile ruler and a depth gauge (here, the authors used a 0.7-mm suction tip, off-label) (C, D)
The implant can be bent in the transition zone, ±90° in the horizontal plane and −30° in the vertical plane (Fig. 5). While bending, the implant should be held with the thumbs and index fingers of both hands, one at the positioning aid and one at the BC-FMT (Figs. 1 and 5). The receiver coil and the attachment magnet are placed in a subperiosteal pocket, directly on the skull. The fixation wings with anchor holes should lay flat on the bone (Fig. 6A, B). When pressing the BC-FMT down with a finger, it should not wiggle or wobble. The BC-FMT is then fixed with self-drilling cortical screws (Fig. 6C, D).
The audio processor retention force is determined by the thickness of the skin over the magnet and receiver coil and by the magnet strength [32]. For the BCI 602, skin thickness should not exceed 7 mm; this can be tested with a skin flap gauge [1]. Skin thickness can also be determined by putting an injection needle through the skin in the area where the receiver coil and magnet will be placed.
Positioning the receiver coil and magnet. A The implant can be bent at the transition zone (white arrow in C) ±90° in the horizontal plane and −30° in the vertical plane. While bending, the implant should be held with the thumbs and index fingers of both hands; one hand should be placed at the positioning aid (B and black arrow in C), and the other hand should be placed at the bone conduction-floating mass transducer. (A: MED-EL, Innsbruck, Austria, with permission)
Fixing the bone conduction-floating mass transducer (BC-FMT) to the bone. A, B The fixation wings with anchor holes should lay flat on the bone. When pressing the BC-FMT down with a finger, it should not wiggle or wobble. C, D The BC-FMT is fixed with self-drilling cortical screws; this procedure is simpler in this model (BCI 602) than in the previous model (BCI 601). (A, C: MED-EL, Innsbruck, Austria, with permission)
By successively moving the drill bit away from the structures mentioned, they can be avoided
Skin closure is typically performed in layers, with resorbable (subcutaneous and pericranial: 3/0) and non-resorbable (skin, 4/0) sutures. Alternatively, cyanoacrylate topical skin adhesive can be used to close the skin. The pericranial layer can usually be closed completely over the BC-FMT, despite its partial elevation above the bone surface.
The first activation and fitting of the audio processor is carried out after complete disappearance of any swelling over the receiver coil and magnet, at approximately 4 weeks after surgery, in an appropriate audiological center. Thereafter, an adaption phase of several weeks is necessary, depending on the individual patient. Technical and medical check-ups are necessary at least once per year.
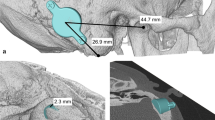
Figure 7 shows the preoperative planning for implanting bone conduction hearing systems in a 4.5-year-old patient with atresia (ear canal stenosis, malformation of the malleus and incus, and a thickened stapes footplate) and complete conductive hearing loss. The parents declined the offer to continue hearing rehabilitation with a soft-band bone conduction hearing aid or canaloplasty with tympanoplasty. Preoperative 3D planning was performed without BC Lifts, since lifts would further elevate the BC-FMT over the bony surface, a feature the authors like to avoid, particularly in the presence of thin skin, which is common in children.
Preoperative plan for the implantation of an active, transcutaneous bone conduction hearing system in a 4.5-year-old patient with atresia and complete conductive loss (according to Plontke et al. 2014 [17]). The bone conduction-floating mass transducers required intracranial penetrations of: (A, B) 6–7 mm for the BCI 601 and (C, D) <2 mm for the BCI 602. Due to the expected skull growth [25], a computed tomography scan and planning were not repeated before surgery at the age of 5.5 years. Intraoperatively, the dura and sigmoid sinus were exposed, but not impressed. This patient is also shown in Fig. 4
The audio processor is first fitted and activated at an audiology center following wound healing
Figure 8 shows the preoperative planning for an adult patient with bilateral complex malformation and after previous hearing rehabilitation with a percutaneous bone conduction hearing implant on the right side (BAHA, Cochlear, Sydney, Australia) and a transcutaneous system on the left side (BCI 601). The patient had experienced recurrent skin irritation around the abutment, including skin overgrowth; therefore, the percutaneous bone conduction system on the right side was replaced with a BCI 602. Detailed preoperative CT-based planning was required due to scars from the previous skin incisions, the plan to simultaneously explant the fixture, and the presence of mastoid hypoplasia. Placing the BC-FMT required limited exposure and temporary impression of the sigmoid sinus (Fig. 9). By pressing the bony island of the sinus inward with a suction tip, it could be protected while the BC-FMT implant bed was molded with the burr. This technique can also be applied to avoid injury to the dura.
Radiological planning and surgical field for the simultaneous explantation of a fixture from a percutaneous bone conduction implant and implantation of a BCI 602 (right ear). A The computed tomography (CT) scout view shows the fixture. Axial CT (B) and coronal (C) CT show mastoid hypoplasia, the lateralized sigmoid sinus, and minimal cortical bone thickness (<5 mm) at the bone conduction-floating mass transducer (BC-FMT) position. The abutment is completely overgrown with skin (black arrow) (D) and is removed before creating the implant bed (E). (A–C: CT images reproduced with permission from Prof. Dr. med. M.A. Weber, Institute for Diagnostic and Interventional Radiology, Pediatric and Neuro-Radiology, University Medicine Rostock)
Placing the bone conduction-floating mass transducer (BC-FMT) close to the sigmoid sinus. A, C Due to the close proximity of the sigmoid sinus (S), a bony island is created on the exposed sinus wall (white arrow). This can be used to protect the sinus from the drill bit by temporarily and gently pressing on the island with the suction tip. B, D Final position of the BC-FMT after removing the pre-existing fixture (asterisk)
Discussion
The dimensions of the BC-FMT are based on physical and technical requirements. The previous model (BCI 601) was associated with considerable risk of depressing the sinus and/or the dura, particularly in children, malformations, poorly pneumatized mastoids, and after canal wall down surgery.
To avoid depressing the sinus or dura, CT-based surgical planning is recommended
Therefore, detailed preoperative, CT-based surgical planning was recommended in those situations [2, 6, 7, 12, 13, 15, 17, 20, 22, 27,28,29,30, 34]. The introduction of BCI Lifts increased the probability that the BC-FMT could be fitted to the bone with 4‑mm lifts in up to 100% of patients older than 9 years [20, 28].
A recent meta-analysis that focused on the BCI 601 showed a rate of 1.7% (five of 289 implantations) for serious adverse events (SAEs), i.e., complications that led to surgical revision or device explantation [14]. A previous retrospective, monocentric observational study reported that the BCI 601 was explanted in five of 64 patients (7.8%) due to implantation out of audiological (one patient) or anatomical indication criteria (four patients). Three patients experienced protrusions into an open mastoid (“radical”) cavity, and one patient received an implantation under the scar tissue from a previous BAHA implant [5]. Another retrospective, multicenter observational study reported SAEs in three of 61 (5%) patients. One patient required inpatient treatment due to a procedure-related wound infection and a history of multiple previous reconstruction surgeries for dysplasia and atresia. The second patient complained of cephalalgia, which, during revision surgery, was found to be due to newly formed soft tissue and bone between the implant and the dura. In the third patient, the device was exchanged due to an unanticipated device-related SAE (device failure) at 27 months after the primary surgery [18].
The lower penetration depth of the BC-FMT into the temporal bone requires a larger BC-FMT diameter
At the authors’ centers, only one of 51 (2%) patients required a BCI 601explantation. However, that was not device-related, but due to sudden hearing loss in the contralateral ear and, thus, the patient lost the audiological indication range for a CROS. Two revision surgeries (3.9%) were necessary; one was to reduce the skin flap over the receiver coil and magnet, and the other was to relocate the implant bed due to progressive outlining of the implant through the preexisting thin retroauricular skin, without skin irritation. Two other patients experienced infections of the auricular episthesis anchors; however, the infections were not related to the bone conduction hearing implant.
The BCI 601 and 602 manufacturer has specified that the audiological indication limit is a maximum bone conduction threshold of 45 dB, in the frequency range of 0.5–to 3 kHz. Considering the maximum power output and a dynamic range of 30–35 dB, and based on the authors’ own experience, the best audiological results (sufficient loudness and dynamic range) are achieved when bone conduction has not yet reached the 45-dB limit [16, 19, 21]. For single-sided deafness, the bone conduction threshold should not exceed 20 dB, in the range of 0.5–3 kHz, in the contralateral (hearing) ear (based on manufacturer specifications and the authors’ own experience).
With the new active BCI and its optimized geometry, the anatomical indication range has significantly increased compared to the previous model (BCI 601). A study that performed “virtual surgery” in 151 temporal bones of 81 children and young adults (ages 5 months–20 years) demonstrated that, in all patients aged 12 years and older, the BCI 602 could be completely fitted to the bone. In patients aged 3–5 years, the BCI 602 could be fitted to the bone in 75% of cases. In contrast, a complete bone fit was not achieved with the BCI 601 in any of the temporal bones without BCI lifts [33]. However, the BCI 602 has not been approved for this age group of up to 5 years. A lower penetration depth would entail a reduced volume of the BC-FMT. However, adequate acceleration of the skull, which is necessary for stimulating the inner ear, would be difficult to achieve. The resonance frequency is indirectly, exponentially related to the mass of the BC-FMT (~m−1/2). Thus, for an electromagnetic transducer with a given mass and resonance frequency, a lower penetration depth required a larger diameter (BCI 601: 15.8 mm and BCI 602: 18.2 mm; Fig. 1). This corresponds to a 15% increase in the diameter and a 45% reduction in the volume of the implant bed. The latter was reached by partially translocating the BC-FMT above the skull surface (Fig. 1). Due to the translocation of the electronics (demodulator) into the BC-FMT, the overall “footprint” of the implant has decreased. Consequently, depending on the individual configuration of the temporal bone, implantation in children under 5 years old appears to be possible and has been performed (off-label) in individual cases (Fig. 10).
Preoperative planning based on Plontke et al. 2014 [17] for the implantation of the active transcutaneous bone conduction hearing systems, BCI 601 and BCI 602, in a 3-year-old patient. The patient had bilateral atresia, poorly pneumatized mastoids, hypoplastic tympanic cavities, dysplastic ossicles, an atypical facial nerve course, and complete conductive loss. Without the BCI Lifts, the BCI 601 required intracranial penetrations of 6.2 mm (BCI 601 right) and 4.4 mm (BCI 601 left). The new implant with optimized geometry (BCI 602) required intracranial penetration, with an impression of the dura and/or sinus, of 2 mm (BCI 602right). However, intracranial penetration was completely avoided on the left side by using a 1-mm BCI Lift at the inferior fixation wing (bottom row center). After informed consent about the off-label use in this age group, and due to the explicit wishes of the parents, the BCI 602 was implanted in this patient at the age of 3 years and 4 months (this patient is also shown in Fig. 3). The axial computed tomography (CT) (center top) and coronal CT (center middle) are reproduced with permission from Prof. Dr. S. Kösling (Radiology, University Medicine Halle, Germany)
Summary
-
The new active bone conduction hearing implant technology is based on a system that has been reliable since 2012.
-
The surgery is feasible, standardized, and safe.
-
The optimized geometric design has improved the fit of the implant to the bone even under challenging anatomical conditions.
References
Anonymous MED-EL Medical Electronics. Bonebridge (BCI 602) Surgical Guide V 2.0 (2019). In: MED-EL, Innsbruck, Austria
Arnold H, Schulze M, Wolpert S et al (2018) Positioning a novel transcutaneous bone conduction hearing implant: a systematic anatomical and radiological study to standardize the retrosigmoid approach, correlating navigation-guided, and landmark-based surgery. Otol Neurotol 39:458–466
Baumgartner WD, Hamzavi JS, Boheim K et al (2016) A new transcutaneous bone conduction hearing implant: short-term safety and efficacy in children. Otol Neurotol 37:713–720
Beutner D, Delb W, Frenzel H et al (2018) Guideline “Implantable hearing aids”-short version : German S2k guideline of the Working Group of German-speaking Audiologists, Neurootologists and Otologists (ADANO), of the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO) in collaboration with the German Society of Audiology (DGA), the German Society of Phoniatrics and Pediatric Audiology (DGPP), and patient representatives. HNO 66:654–659
Brkic FF, Riss D, Scheuba K et al (2019) Medical, technical and audiological outcomes of hearing rehabilitation with the bonebridge transcutaneous bone-conduction implant: a single-center experience. J Clin Med 8:1614. https://doi.org/10.3390/jcm8101614
Canis M, Ihler F, Blum J et al (2013) CT-assisted navigation for retrosigmoidal implantation of the Bonebridge. HNO 61(12):1038–1044
Cho B, Matsumoto N, Mori M et al (2014) Image-guided placement of the bonebridge without surgical navigation equipment. Int J Comput Assist Radiol Surg 9:845–855
Edmiston RC, Aggarwal R, Green KM (2015) Bone conduction implants—a rapidly developing field. J Laryngol Otol 129:936–940
Frenzel H, Hanke F, Beltrame M et al (2010) Application of the vibrant soundbridge in bilateral congenital atresia in toddlers. Acta Otolaryngol 130:966–970
Fussey JM, Harterink E, Gill J et al (2018) Clinical outcomes following Cochlear BIA300 bone anchored hearing aid implantation in children. Int J Pediatr Otorhinolaryngol 111:89–92
Kiringoda R, Lustig LR (2013) A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol 34:790–794
Kong TH, Park YA, Seo YJ (2017) Image-guided implantation of the Bonebridge with a surgical navigation: a feasibility study. Int J Surg Case Rep 30:112–117
Law EK, Bhatia KS, Tsang WS et al (2016) CT pre-operative planning of a new semi-implantable bone conduction hearing device. Eur Radiol 26:1686–1695
Magele A, Schoerg P, Stanek B et al (2019) Active transcutaneous bone conduction hearing implants: systematic review and meta-analysis. PLoS ONE 14:e221484
Matsumoto N, Takumi Y, Cho B et al (2015) Template-guided implantation of the Bonebridge: clinical experience. Eur Arch Otorhinolaryngol 272:3669–3675
Mertens G, Desmet J, Snik AF et al (2014) An experimental objective method to determine maximum output and dynamic range of an active bone conduction implant: the Bonebridge. Otol Neurotol 35:1126–1130
Plontke SK, Radetzki F, Seiwerth I et al (2014) Individual computer-assisted 3D planning for surgical placement of a new bone conduction hearing device. Otol Neurotol 35:1251–1257
Rader T, Stover T, Lenarz T et al (2018) Retrospective analysis of hearing-impaired adult patients treated with an active transcutaneous bone conduction implant. Otol Neurotol 39:874–881
Rahne T, Plontke SK (2016) Device-based treatment of mixed hearing loss: an audiological comparison of current hearing systems. HNO 64:91–100
Rahne T, Schilde S, Seiwerth I et al (2016) Mastoid dimensions in children and young adults: consequences for the geometry of transcutaneous bone-conduction implants. Otol Neurotol 37:57–61
Reinfeldt S, Hakansson B, Taghavi H et al (2015) New developments in bone-conduction hearing implants: a review. Med Devices 8:79–93
Reinfeldt S, Ostli P, Hakansson B et al (2015) Study of the feasible size of a bone conduction implant transducer in the temporal bone. Otol Neurotol 36:631–637
Salcher R, Zimmermann D, Giere T et al (2017) Audiological results in SSD with an active transcutaneous bone conduction implant at a retrosigmoidal position. Otol Neurotol 38:642–647
Sardiwalla Y, Jufas N, Morris DP (2018) Long term follow-up demonstrating stability and patient satisfaction of minimally invasive punch technique for percutaneous bone anchored hearing devices. J Otolaryngol Head Neck Surg 47:71
Schilde S, Plontke SK, Rahne T (2017) A three-dimensional geometric-morphometric study to quantify temporal bone growth and its consequences for the success of implanting bone anchored hearing devices. Otol Neurotol 38:721–729
Sprinzl GM, Wolf-Magele A (2016) The Bonebridge bone conduction hearing implant: indication criteria, surgery and a systematic review of the literature. Clin Otolaryngol 41:131–143
Takumi Y, Matsumoto N, Cho B et al (2014) A clinical experience of ‘STAMP’ plate-guided Bonebridge implantation. Acta Otolaryngol 134:1042–1046
Thomas JP, Van Ackeren K, Dazert S et al (2018) Transmastoid implantability of an active transcutaneous bone conduction implant in adults with regard to the underlying pathology: a radiological simulation study. Acta Otolaryngol 138:530–536
Todt I, Lamecker H, Ramm H et al (2014) A computed tomographic data-based vibrant bonebridge visualization tool. Cochlear Implants Int 15(Suppl 1):S72–S74
Todt I, Lamecker H, Ramm H et al (2014) Development of a computed tomography data-based Vibrant Bonebridge viewer. HNO 62:439–442
Vickers D, Canas A, Degun A et al (2018) Evaluating the effectiveness and reliability of the vibrant soundbridge and Bonebridge auditory implants in clinical practice: study design and methods for a multi-centre longitudinal observational study. Contemp Clin Trials Commun 10:137–140
Wagner L, Honig E, Frohlich L et al (2019) Optimal retention force of audio processor magnets. Otol Neurotol 40:e482–e487
Wenzel C, Schilde S, Plontke SK et al (2020) Changes in bone conduction implant geometry improve the bone fit in healthy mastoids of children and young adults. Otol Neurotol [in press]
Wimmer W, Gerber N, Guignard J et al (2015) Topographic bone thickness maps for Bonebridge implantations. Eur Arch Otorhinolaryngol 272:1651–1658
Zernotti ME, Sarasty AB (2015) Active bone conduction prosthesis: Bonebridge(TM). Int Arch Otorhinolaryngol 19:343–348
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.K. Plontke: Audio Cure Pharma GmbH, Berlin (consultancy); Oticon Medical, Denmark; Cochlear Ltd., Australia; Federal Ministry of Research and Education (Research Projects); BV-HNOe.V.; MerckSerono; Infectopharm; Dr.Willmar Schwabe GmbH& Co. KG, Deutschland (honrary for lectures and travel support for lectures). T. Rahne: Oticon Medical,Denmark; Cochlear Ltd., Australia (Research Projects). S.K. Plontke, G. Götze, C. Wenzel, T. Rahne, and R.Mlynski: MED-EL, Austria, and MED-EL Germany (Research Projects); MED-EL Austria and MED-EL Germany (travel support for lectures).
All hearing rehabilitation procedures occured as routine treatment, after individual informed consent, and according to current ethical guidelines. The “off-label-use” of the implant in one patient (Figs. 3 and 10) occured after special informed consent and on the specific request of the parents.
The supplement containing this article is not sponsored by industry.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Plontke, S.K., Götze, G., Wenzel, C. et al. Implantation of a new active bone conduction hearing device with optimized geometry. HNO 68 (Suppl 2), 106–115 (2020). https://doi.org/10.1007/s00106-020-00877-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00106-020-00877-2