Abstract
Background
Vestibular evoked myogenic potentials (VEMPs) are increasingly being used for testing otolith organ function.
Objective
This article provides an overview of the anatomical, biomechanical and neurophysiological principles underlying the evidence-based clinical application of ocular and cervical VEMPs (oVEMPs and cVEMPs).
Material and methods
Systematic literature search in PubMed until April 2019.
Results
Sound and vibration at a frequency of 500 Hz represent selective vestibular stimuli for the otolith organs. The predominant specificity of oVEMPs for contralateral utricular function and of cVEMPs for ipsilateral saccular function is defined by the different central projections of utricular and saccular afferents. VEMPs are particularly useful in the diagnosis of superior canal dehiscence and otolith organ specific vestibular dysfunction and as an alternative diagnostic approach in situations when video oculography is not possible or useful.
Conclusion
The use of VEMPs is a simple, safe, reliable and selective test of dynamic function of otolith organs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vestibular evoked myogenic potentials (VEMPs) are short-latency, otolith-driven reflexes elicited by air-conducted sound (ACS), bone-conducted vibration (BCV), or galvanic vestibular stimulation and recorded from ocular (oVEMPs) or cervical (cVEMPs) muscles [5, 71, 74]. Combining VEMPs with the video head impulse test (vHIT) [36] allows for a receptor-specific examination of all five vestibular endorgans in clinical practice (oVEMPs: utricle; cVEMPs: saccule; vHIT: semicircular canals; [14]).
Reflex pathways
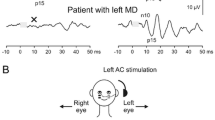
Ocular VEMPs (oVEMPs) are mediated by a predominantly crossed reflex pathway projecting from the utricle to the ipsilateral vestibular nuclei and via the medial longitudinal fasciculus to the contralateral oculomotor nucleus (N. III) that supplies the contralateral inferior oblique muscle (Fig. 1a). From there, an early excitatory potential (n10) is recorded by surface electrodes approximately 10 ms after stimulus onset while the subject is looking upwards (Fig. 1b; [17, 25]).
The oVEMP n10 amplitude predominantly reflects contralateral utricular function.
Reflex pathways and VEMPs recorded in a–c a healthy subject and d–f a patient with right-sided unilateral vestibular loss (uVL). III oculomotor nucleus; XI spinal accessory nucleus; black hexagon inhibitory interneurons in the vestibular nuclei projecting to the motoneurons of the ipsilateral sternocleidomastoid muscle (SCM). a–c In a healthy subject, symmetric oVEMP n10 responses are recorded from the inferior oblique muscle beneath the left and right eyes. Likewise, symmetric cVEMP p13n23 responses are present in the left and right SCM. d–f In a patient with right-sided uVL (X), however, contralateral (= left) oVEMPs (crossed reflex pathway) and ipsilateral (= right) cVEMPs (uncrossed reflex pathway) are absent. VEMPs vestibular evoked myogenic potentials, c cervical, o ocular, inf inferior. (Slightly modified and reprinted from [17] with permission from © John Wiley & Sons)
The oVEMP n10 amplitude predominantly reflects contralateral utricular function
In contrast, cVEMPs involve a mainly uncrossed reflex circuit running from the saccule to the ipsilateral vestibular nuclei from where inhibitory interneurons project to the ipsilateral spinal accessory nucleus (N. XI) that innervates the sternocleidomastoid muscle (SCM). The early inhibitory potential (p13n23) recorded from the activated SCM between 13 and 23 ms after stimulus onset is an indicator of predominantly ipsilateral saccular function (Fig. 1c; [17]).
The cVEMP p13n23 amplitude is predominantly an indicator of ipsilateral saccular function.
The cVEMP p13n23 amplitude is predominantly an indicator of ipsilateral saccular function
VEMPs are vestibular—and not cochlear—reflexes. They are preserved in subjects with profound sensorineural hearing loss, but intact peripheral vestibular function. On the other hand, they are absent in patients after vestibular neurectomy with normal hearing [5, 41,42,43, 71]. While sensorineural hearing loss does not affect VEMPs, it should however be noted that a conductive hearing loss (air–bone gap) as small as 10 dB may cancel ACS-evoked VEMP responses completely ([86]; for details see the section “Nature of the stimulus”).
Relevant anatomy and physiology of the otolith organs
The otolith (macula) organs sense linear acceleration, the utricle predominantly in the horizontal plane and the saccule predominantly in the vertical plane (Fig. 2a; [58, 69]). During constant or low-frequency linear acceleration, the otolith organs work as accelerometers, i.e., the otoconial membrane lags behind the underlying neuro-epithelium due to the inertia of the otoconia, thus, causing a relative movement between the two layers that is opposite to the direction of linear acceleration (Figs. 2b, c and 3a; [21, 34]). The resulting shearing force deflects the hair cell bundles of vestibular hair cells in the neuro-epithelial layer. Deflection of the stereocilia towards the kinocilium results in depolarization of the hair cell, thus, triggering the signal transduction process between the vestibular hair cell and its postsynaptic afferent nerve fiber.
Schematic diagrams of a the otolith organs, b,c their vestibular hair cells and d their afferent innervation. a Spatial orientation of the utricular and saccular maculae in the labyrinth. Dashed lines: lines of polarity reversal/striola. Arrows: polarization vectors of the vestibular hair cells. For details see [69]. b Amphora-shaped type I vestibular hair cell (with calyx synapse) and cylindrical type II vestibular hair cell (with bouton synapse) and the overlying otoconia at rest: no deflection of hair cell stereocilia. c Vestibular hair cells and otoconia during constant or low-frequency linear acceleration: relative motion of the otoconial membrane relative to the neuroepithelial layer and deflection of hair cell stereocilia opposite to the direction of linear acceleration. (Slightly modified and reprinted from [21] with permission from © I.S. Curthoys et al., CC BY 4.0 [https://creativecommons.org/licenses/by/4.0/]). d Afferent innervation of the vestibular organ. Yellow: superior vestibular nerve; blue: inferior vestibular nerve; SG Scarpa’s ganglion; ant./hor./post. SCC anterior (= superior)/horizontal/posterior semicircular canal. (Reprinted from [17] with permission from © John Wiley & Sons)
Dual mode of action of the otolith organs as a accelerometers for constant and low-frequency linear accelerations (= sustained channel of otolithic function) and b seismometers for high-frequency changes in acceleration (= transient channel). (Reprinted from [21] with permission from © I.S. Curthoys et al., CC BY 4.0, https://creativecommons.org/licenses/by/4.0)
Otolith afferents are organized in two different functional channels [29, 31]. The so-called transient (= dynamic) system, which is particularly sensitive to changes in linear acceleration (“jerks”), receives its input from highly specialized type I striolar vestibular hair cells that are connected to postsynaptic vestibular afferents with irregular resting activity via ultrafast calyx synapses (Fig. 2b, c; for more details see S1 [online supplementary material] and [9, 76]). On the other hand, constant and low-frequency linear accelerations are processed by the sustained (= transient) channel that involves mainly type II vestibular hair cells and regular vestibular afferents from the extrastriola [20, 21, 23, 26].
While all utricular afferents run in the superior vestibular nerve, 90% of saccular afferents are organized in the inferior vestibular nerve and 10% in the superior vestibular nerve. The so-called “Voit’s nerve” contains mainly afferents from the hook region of the saccular macula (Fig. 2d; [24]).
Sound and vibration as otolithic stimuli
In clinical practice, usually sound and vibration of 500 Hz and above are applied to evoke VEMPs. How can these—with regard to the vestibular system—high frequencies provide adequate linear acceleration for the otolith organs, a seemingly sluggish system whose function is based on the inertia of the otoconia?
Biomechanical evidence
Each cycle of a sound wave (ACS or BCV) is able to create a pressure wave—and thus a change in acceleration—in the endolymph surrounding the otolith maculae (for details see S2 [online supplementary material]). Accordingly, a stimulus frequency of 500 Hz is equivalent to 500 changes in acceleration per second, making it an ideal “jerk” stimulus for the transient vestibular system [21]. The conventional “accelerometer” mode of the otolith organs (Fig. 3a) would, however, be too sluggish to encode this high-frequency acceleration with adequate temporal precision. Instead, the utricle and the saccule work as seismometers in this situation—similar to the mechanism by which technical seismometers detect earthquakes (see Infobox 1 for further information about the mode of action of a seismometer): the neuro-epithelium oscillates with the stimulus frequency, while the otoconia remain stationary because of their inertia (Fig. 3b; [34]) resulting in a relative motion between the otoconial membrane and the neuro-epithelium, albeit with reversed roles of the two layers as compared to the accelerometer mode. Finally, this relative motion causes deflection of the hair cell stereocilia and, thus, hair cell depolarization (see above and [21]).
Experimental evidence for the “seismometer” mode of otolith organs derives from a sophisticated guinea pig model showing that application of sound or vibration to the guinea pig’s skull resulted in periodic oscillations of the utricular macula and de-/repolarizations of the utricular hair cells in sync with the stimulus frequency up to several kHz (for details see S3 [online supplementary material] and [65, 66]).
It is the dual mode of action as accelerometers and seismometers that allows the otolith organs to encode both constant/low-frequency linear acceleration and high-frequency sound and vibration. The 500 Hz ACS or BCV stimulus applied in clinical VEMP testing is ideally suited to activate the transient (= dynamic) otolithic system [21].
Neurophysiological evidence
How does sound- and vibration-induced depolarization of vestibular hair cells activate vestibular afferents? And which types of afferents: otolith or semicircular canal? Those with irregular or regular resting discharge? The answers to these questions were mainly found by performing extracellular recordings of primary vestibular neurons in Scarpa’s ganglion (SG) during application of ACS or BCV to a guinea pig’s skull (Fig. 2d). The measurement set-up, in particular the stimulus parameters, were chosen to match the conditions during VEMP testing in humans [10, 13, 15, 18, 21, 22].
These experiments revealed that 500 Hz sound and vibration selectively activate type I vestibular hair cells in the utricular and saccular striola and their postsynaptic vestibular afferents with irregular resting discharge (Fig. 4). The combination of type I vestibular hair cells, ultrafast calyx synapses, and irregular otolith afferents is ideally suited for a stimulus- and phase-locked coding of these dynamic vestibular stimuli (500 changes in acceleration per second) with high temporal precision (see above and S1, S2 [online supplementary material] for more details).
In contrast, the resting activity of semicircular canal (both regular and irregular) and regular otolith afferents is virtually not altered during application of 500 Hz ACS or BCV at stimulus levels usually employed for clinical VEMP testing. Most probably, the sound- and vibration-induced endolymph flow in the semicircular canals is too weak to deflect hair cell stereocilia in the canal crista as long as the bony labyrinth is intact [75]. This may however change when a third mobile window is introduced into the bony wall of the inner ear in addition to the round and oval windows (see section “Superior canal dehiscence” and S4 [online supplemental material] for details).
Response of an utricular afferent neuron with irregular resting discharge to 500 Hz bone-conducted vibration (BCV) and air-conducted sound (ACS) in the guinea pig: both BCV and ACS cause a stimulus-locked increase in spike discharge rate in the irregular utricular afferent neuron. (Modified from [13])
Neuronal projections
If sound and vibration are able to activate both utricular and saccular afferents, how can ocular and cervical VEMPs differentiate between utricular and saccular function? The predominant specificity of oVEMPs for utricular and cVEMPs for saccular function is not due to different stimulus qualities (i.e., ACS and BCV), but to the differential neuronal projections of utricular and saccular afferents in the central vestibular system.
Anatomical and neurophysiological evidence shows that utricular afferents project predominantly to vestibulo-ocular neurons in the vestibular hindbrain, while saccular afferents mainly contact vestibulo-spinal neurons [32, 40, 59, 77,78,79]. Therefore, ocular VEMPs recorded from the inferior oblique eye muscle predominantly reflect utricular function, and cervical VEMPs obtained from the SCM are predominantly an indicator of saccular function (Fig. 1a–c; [14, 17]).
At this point, it is important to notice that the separation between the central projections of utricular and saccular afferents is predominant, and not complete. Furthermore, 10% of the saccular afferents travel in the superior vestibular nerve together with the utricular afferents (see above and Fig. 2d). A number of clinical studies in patients with superior and inferior vestibular neuritis indicate, however, that the predominant specificity of oVEMPs for utricular and cVEMPs for saccular function is sufficient for distinguishing between utricular and saccular dysfunction in clinical practice (see also section “Vestibular neuritis” and [6, 11]).
To make things even more complicated, not only otolith, but also semicircular canal afferents project to vestibulo-ocular and vestibulo-spinal neurons in the vestibular hindbrain. For instance, afferents of the superior semicircular canal provide excitatory input to the contralateral inferior oblique muscle and inhibitory input to the ipsilateral SCM via central vestibular neurons [78, 79]. These pathways are however silent in subjects with a normally encased bony labyrinth, as the fluid displacement in the superior semicircular canal during application of 500 Hz sound and vibration is too small to activate the respective vestibular hair cells and afferents (see above). The “silent” projections may, however, become activated if a third mobile window is introduced into the bony labyrinth, e.g., in superior canal dehiscence, resulting in increased VEMP responses (see below and S4 [online supplemental material]).
Measurement set-up and analysis of data
This section summarizes the most important principles concerning measurement set-up and data analysis of VEMP recordings. For more details, please refer to [26, 63, 74].
Stimulus quality
Short ACS and BCV stimuli can be used for recording both o‑ and cVEMPs (for detailed stimulus parameters see below and [63, 68, 74]). A stimulus frequency of 500 Hz is generally recommended for most clinical applications because most of the neurophysiological data in the guinea pig model were obtained for 500 Hz sound or vibration [21, 74]. Please note that stimulus frequencies <500 Hz are able to activate irregular semicircular canal in addition to irregular otolith afferents—even in the normally encased bony labyrinth. As this might compromise the otolithic specificity of VEMPs, these frequencies are not recommended for VEMP testing in clinical practice [19].
Beside sound and vibration, galvanic currents applied to the mastoid via large surface electrodes can be employed as VEMP stimuli (galvanic or gVEMPs). So far, this technique has mainly been applied in experimental settings, and not in clinical routine [28].
Air-conducted sound: ACS (clicks or tone bursts) is presented to both ears sequentially with calibrated headphones or insert phones, while the ipsilateral cVEMP or the contralateral oVEMP response is recorded (Fig. 5). The test subject has to be protected from excessive sound exposure during presentation of ACS stimuli. When using ACS, the test subject has to be protected from excessive sound exposure.
When using ACS as a VEMP stimulus, the test subject has to be protected from excessive sound exposure
To achieve this aim, a careful calibration of the sound source is of paramount importance. As a rule of thumb, a sound pressure level (SPL) of 130 dB(A) applied for 1 s is regarded to be safe. The peak (p) SPL should not exceed 140 dB(A). Detailed information concerning the calculation of sound exposure and prevention of noise trauma has been provided by [7, 8, 68, 74]. If the sound pressure level is indicated in “dB nHL” (normal hearing level) by the manufacturer of the VEMP platform, it has to be converted into “dB pSPL” or “pe (peak equivalent) SPL” in order to calculate safe sound exposure levels. The individual conversion factor depends on multiple variables, such as headphone type, stimulus parameters (see below), and ear canal volume [39, 68]. Conversion factors for the most common test conditions are often provided in the manufacturer’s instructions (e.g., [38]), otherwise they have to be determined during calibration of the sound source.
The use of ACS stimuli requires functional integrity of the middle ear.
As a rule of thumb, a 500 Hz ACS tone burst of 95–100 dB nHL is required to obtain stable cVEMPs in a healthy subject, while 5–10 dB more are needed for oVEMPs, reflecting the higher sensitivity of irregular saccular as compared to utricular afferents to ACS in the guinea pig model [18, 72, 74].
Finally, the neurotologist should always be aware that the use of ACS stimuli requires intact middle ear function. An air–bone gap as small as 10 dB is able to absorb so much sound energy in the middle ear that the remaining energy finally reaching the labyrinth might be too small to evoke reliable VEMP responses [86].
The use of ACS stimuli requires functional integrity of the middle ear
Bone-conducted vibration: BCV is usually applied to the midline of the forehead at the hairline (Fz; Fig. 5a), from where the vibration propagates through the skull and causes simultaneous linear acceleration in both labyrinths with approximately equal intensity. Therefore, a time-saving simultaneous recording of right- and left-sided VEMP responses is possible in this set-up [42]. Furthermore, BCV does not put the test subject at risk of excessive sound exposure, as irregular otolith afferents are much more sensitive to BCV (thresholds of ~0.02 g) than ACS (thresholds >80 dB SPL) [21]. Vibration can also be applied in patients with conductive hearing loss, as the sound energy is transported to the labyrinth via bone and connective tissue, and not through the middle ear.
A conventional bone-conduction device (e.g., RadioEar B71) is usually too weak for application at Fz; therefore, more powerful electromechanical vibrators are recommended (e.g., minishaker type 4810, Bruel and Kjaer) along with an appropriate amplifier (e.g., power amplifier type 2718, Bruel and Kjaer) [41, 42]. It should however be noted that the latter are not certified medical devices; therefore, their use is currently restricted to scientific applications. Tapping Fz with a standard reflex hammer (which is a medical device) is a cost-effective alternative for delivering BCV to Fz in clinical practice (for technical details see [37, 41, 49]).
Stimulus parameters
The amplitude of a VEMP response is not determined by the total duration of the stimulus, but by its abrupt start (“jerk”; [3, 48]). The latter is the adequate stimulus for activating the transient channel of otolithic function (see above). Therefore, short stimuli (up to 6 ms) with short rise times (0–2 ms) are generally recommended for evoking o‑ and cVEMPs in clinical practice. In general, 50–200 stimuli at a repetition rate of 5 pulses per second (pps) are required to obtain robust VEMP responses [74]. When using the reflex hammer, 10 taps to Fz are usually sufficient to evoke a stable signal, as this is an ideal “jerk” with a rise time of 0 ms [17, 37].
Placement of recording electrodes
For recording of oVEMPs, the active electrode is placed on the skin covering the inferior oblique muscle, i.e., on the infraorbital rim in one line with the pupil, the reference electrode is located 1–2 cm further below (Fig. 5; [42]). The patient is asked to look upwards (“look at your forehead”, Fig. 5a) during the recording as this increases the relatively small oVEMP n10 amplitude (5–10 µV). This effect is caused by a combination of bringing the belly of the inferior oblique muscle closer to the recording electrode on the skin and increasing the tonic activity of the muscle during upward gaze [73].
The active electrode for recording cVEMPs is placed on the skin above the mid-third of the anterior arm of the SCM, the reference electrode is located either on the medial end of the clavicula (Fig. 5b) or on the sternum (in case only one reference electrode is used for the two sides). The ground electrode for both o‑ and cVEMPs can be fixed to the chin or sternum. A certain baseline activity of the SCM is required for recording cVEMPs, as these are inhibitory responses. To this end, the test subject is asked to lift their head straight ahead while lying in a semi-recumbent position on the examination table. If the baseline activity of the SCM is too low with this maneuver, the test subject is asked to lift the head and turn it to the contralateral side [74].
Outcome parameters
Amplitudes and latencies of the oVEMP n10 and the cVEMP p13n23 responses are the most important outcome parameters in clinical practice. For a better comparison between right- and left-sided amplitudes, the asymmetry ratio (AR) is calculated:
An AR value >40% indicates an asymmetry between right- and left-sided dynamic otolithic function [42, 43, 86].
The “raw” cVEMP p13n23 amplitude (in µV) correlates linearly with the baseline activity of the SCM (in µV) [5]. Therefore, it is recommended to calculate a dimensionless “corrected” p13n23 amplitude: the raw amplitude (obtained from the unrectified averaged trace of the electromyograhic [EMG] recording) is divided by the baseline activity of the SCM. The latter is determined as the average amplitude of the rectified or root mean square (RMS) EMG recorded in parallel to the unrectified EMG in the interval between −20 ms and stimulus onset (0 ms). For details, see Figs. 5 and 6 in [74]. The corrected p13n23 amplitude allows for a better comparison of cVEMP responses recorded from different sides, in different individuals and during different recording sessions.
Normal values
Normal values for VEMPs depend on many variables, such as the test subject’s age and various stimulus parameters (e.g., frequency, intensity, duration, rise times) [1, 3, 60, 67, 72, 85]. Therefore, it is strongly recommended that each neurotological center defines its own normal values for each set of stimulus parameters and different age groups [74].
Clinical applications
The following section focuses on characteristic VEMP responses in peripheral vestibular disorders that have contributed to a better understanding of the neurophysiology behind VEMPs or that provide additional information as compared to other vestibular tests. Detailed accounts of clinical VEMP applications have been provided by, for example [14, 26, 46, 50, 57, 62, 70, 74, 81, 84].
Vestibular neuritis
In line with the afferent innervation of the labyrinth (Fig. 2d), patients with a superior vestibular neuritis display reduced oVEMP n10 amplitudes on the contralateral side (Fig. 1e), while right- and left-sided cVEMPs are symmetrical. On the other hand, reduced ipsilateral cVEMP p13n23 amplitudes and symmetrical oVEMP n10 responses are a hallmark of inferior vestibular neuritis (Fig. 1f; [12, 33, 44, 51, 52, 56, 61, 64, 82]). This “double dissociation” of o‑ and c‑VEMPs in superior versus inferior vestibular neuritis has two important implications. First, it indicates that the two otolith-driven reflexes do not originate in the same subset of vestibular receptors. If they did, one would expect that either both responses or none at all would be affected in superior/inferior neuritis. Second, the double dissociation shows that the predominant specificity of oVEMPs for contralateral utricular function and cVEMPs for ipsilateral saccular function is sufficient to distinguish between utricular and saccular dysfunction in clinical practice—although the neuronal projections of utricular and saccular afferents are not completely separated [11].
Otolith organ specific vestibular dysfunction
VEMPs are an efficient and reliable tool for detecting isolated disorders of the otolith organs [16, 30]. If vestibular testing assesses only semicircular canal function (e.g., by calorics or vHIT), these disorders may easily been missed and classified as “non-vestibular” or “functional”. Therefore, the results of semicircular canal testing have to be complemented by selective tests of otolithic function such as VEMPs in clinical practice.
VEMPs are an efficient and reliable tool for diagnosing otolith organ specific vestibular dysfunction
This is especially important in patients with mild traumatic brain injury or blast trauma who often suffer from isolated dysfunction of the otolith organs despite intact semicircular canal function [2, 47].
Superior canal dehiscence
A dehiscence in the bony roof of the superior semicircular canal creates a third mobile window in the bony labyrinth (beside the oval and round windows) resulting, e.g., in sound-induced vertigo and nystagmus (Tullio phenomenon; for details see Infobox 1 and S4 [online supplementary material]; [35, 83]).
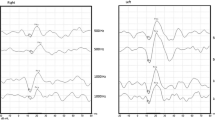
A highly increased oVEMP n10 response recorded from the inferior oblique muscle is a powerful indicator for a superior canal dehiscence (SCD) in the contralateral labyrinth with a sensitivity and specificity >90% depending on stimulus parameters and control populations (Fig. 6a; [45, 54, 80, 88]). Furthermore, patients with an SCD display a contralateral oVEMP n10 response to 4000 Hz ACS or BCV, which is absent in normal subjects with an intact bony labyrinth (Fig. 6b; diagnostic accuracy = 100%). As the latter test does not depend on comparison between right- and left-sided responses, it is ideally suited to diagnose bilateral SCD [55].
Effect of a superior canal dehiscence (SCD) on ocular vestibular evoked myogenic potentials (oVEMPs). a Enhanced contralateral oVEMP n10 amplitude (arrowhead) during application of 500 Hz bone-conducted vibration (BCV) at Fz (midline of the forehead at the hairline). (Reprinted from [54] with permission from © Wolters Kluwer Health, Inc.). b Presence of a contralateral oVEMP n10 response to 4000 Hz BCV and air-conducted sound (ACS) in a case of SCD. (Slightly modified and reprinted from [55] with permission from © SAGE Publications)
The tremendous effect of SCD on the oVEMP n10 amplitude can be explained by activation of the otherwise “silent” vestibulo-ocular projections of superior canal afferents (see section “Neuronal projections”). While semicircular canal hair cells and afferents do not respond to sound or vibration in the normally encased labyrinth, the third mobile window in SCD induces an endolymph flow in the superior semicircular canal [75] finally resulting in activation of type I vestibular hair cells and their postsynaptic irregular afferents. As these project to vestibulo-ocular neurons—just like utricular afferents—they increase the oVEMP n10 amplitude recorded from the inferior oblique eye muscle [4, 27, 79].
Following closure of the superior canal dehiscence in a guinea pig model of SCD, superior semicircular canal afferents do no longer respond to ACS and BCV, which is in line with the normalization of VEMP responses after successful surgical closure of SCD in humans [27, 87].
The “difficult” patient
Most neurotological tests, such as caloric irrigation and vHIT, infer vestibular (dys‑)function from the video-oculographic (VOG) assessment of the vestibulo-ocular reflex, or put simply: you have to look into your patients’ eyes to determine their balance function. This may, however, be difficult or even impossible in certain groups of patients, e.g., those with congenital/infantile nystagmus (superimposition of the VOG recording by the infantile nystagmus), extremely poor vision (blind nystagmus; inability to fixate a target during vHIT), or reduced ability to cooperate (e.g., blink and lid artifacts). In these cases, o‑ and cVEMPs offer a simple, quick, and reliable alternative to obtain some basic information about superior (oVEMPs) and inferior vestibular nerve (cVEMPs) function (Fig. 2d; [14]). Both responses can be recorded in patients with infantile nystagmus [53]. Due to the short duration of the examination (40 s for a trial with 200 stimuli at 5 pps), VEMPs are also a suitable diagnostic tool for patients with limited ability to cooperate, e.g., children and patients with cognitive impairment.
Infobox 1 Background information
Websites accessed on 23 April 2020
-
mode of function of a seismometer: https://www.iris.edu/hq/inclass/animation/seismograph_horizontal
-
animation of the Tullio phenomenon:
Practical conclusion
-
VEMPs (vestibular evoked myogenic potentials) are a simple, safe, reliable, and selective test of transient (= dynamic) otolithic function.
-
500 Hz ACS (air-conducted sound) and BCV (bone-conducted vibration) selectively activate type I vestibular hair cells and irregular otolith afferents in the utricular and saccular striola. The predominant specificity of oVEMPs (ocular vestibular evoked myogenic potentials) for contralateral utricular function and of cVEMPs (cervical vestibular evoked myogenic potentials) for ipsilateral saccular function is due to the differential projections of utricular and saccular afferents to vestibulo-ocular and vestibulo-spinal neurons, respectively.
-
When using ACS, the test subject has to be protected from excessive sound exposure.
-
The “raw” cVEMP p13n23 amplitude is corrected for the baseline activity of the sternocleidomastoid muscle determined by the rectified or root mean square EMG electromyogram for a better comparison between cVEMP responses from different recordings and test subjects.
-
A highly increased oVEMP n10 amplitude is a diagnostic indicator for a contralateral superior canal dehiscence.
-
Informative VEMP recordings can be obtained from patients with congenital/infantile nystagmus, extremely poor vision or limited ability to cooperate during vestibular testing.
Abbreviations
- ACS:
-
Air-conducted sound
- AR:
-
Asymmetry ratio
- BCV:
-
Bone-conducted vibration
- c:
-
Cervical
- Fz:
-
Midline of the forehead at the hairline
- g:
-
Gravitational acceleration
- nHL:
-
Normal hearing level
- o:
-
Ocular
- p:
-
Peak
- pe:
-
Peak equivalent
- pps:
-
Pulses per second
- RMS:
-
Root mean square
- SCD:
-
Superior canal dehiscence
- SCM:
-
Sternocleidomastoid muscle
- SPL:
-
Sound pressure level
- VEMPs:
-
Vestibular evoked myogenic potentials
- vHIT:
-
Video head impulse test
- VOG:
-
Video-oculography
References
Agrawal Y, Zuniga MG, Davalos-Bichara M et al (2012) Decline in semicircular canal and otolith function with age. Otol Neurotol 33:832–839
Akin FW, Murnane OD, Hall CD, Riska KM (2017) Vestibular consequences of mild traumatic brain injury and blast exposure: a review. Brain Inj 31:1188–1194
Burgess AM, Mezey LE, Manzari L et al (2013) Effect of stimulus rise-time on the ocular vestibular-evoked myogenic potential to bone-conducted vibration. Ear Hear 34:799–805
Carey JP, Hirvonen TP, Hullar TE et al (2004) Acoustic responses of vestibular afferents in a model of superior canal dehiscence. Otol Neurotol 25:345–352
Colebatch JG, Halmagyi GM, Skuse NF (1994) Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 57:190–197
Colebatch JG (2010) Sound conclusions? Clin Neurophysiol 121:124–126
Colebatch JG, Rosengren SM (2014) Safe levels of acoustic stimulation: comment on “Effects of acoustic stimuli used for vestibular evoked myogenic potential studies on the cochlear function”. Otol Neurotol 35:932–933
Colebatch JG, Rosengren SM (2016) Safe levels of acoustic stimulation for VEMPs: comment on “Sudden bilateral hearing loss after cervical and ocular vestibular evoked myogenic potentials”. Otol Neurotol 37:117–118
Contini D, Price SD, Art JJ (2017) Accumulation of K(+) in the synaptic cleft modulates activity by influencing both vestibular hair cell and calyx afferent in the turtle. J Physiol 595:777–803
Curthoys IS, Kim J, McPhedran SK, Camp AJ (2006) Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res 175:256–267
Curthoys IS (2010) A balanced view of the evidence leads to sound conclusions. A reply to J.G. Colebatch “Sound conclusions?”. Clin Neurophysiol 121:977–978
Curthoys IS, Iwasaki S, Chihara Y et al (2011) The ocular vestibular-evoked myogenic potential to air-conducted sound; probable superior vestibular nerve origin. Clin Neurophysiol 122:611–616
Curthoys IS, Vulovic V (2011) Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp Brain Res 210:347–352
Curthoys IS (2012) The interpretation of clinical tests of peripheral vestibular function. Laryngoscope 122:1342–1352
Curthoys IS, Vulovic V, Sokolic L, Pogson J, Burgess AM (2012) Irregular primary otolith afferents from the guinea pig utricular and saccular maculae respond to both bone conducted vibration and to air conducted sound. Brain Res Bull 89:16–21
Curthoys IS, Manzari L (2013) Otolithic disease: clinical features and the role of vestibular evoked myogenic potentials. Semin Neurol 33:231–237
Curthoys IS, Vulovic V, Burgess AM et al (2014) Neural basis of new clinical vestibular tests: otolithic neural responses to sound and vibration. Clin Exp Pharmacol Physiol 41:371–380
Curthoys IS, Vulovic V, Burgess AM, Sokolic L, Goonetilleke SC (2016) The response of guinea pig primary utricular and saccular irregular neurons to bone-conducted vibration (BCV) and air-conducted sound (ACS). Hear Res 331:131–143
Curthoys IS (2017) The new vestibular stimuli: sound and vibration-anatomical, physiological and clinical evidence. Exp Brain Res 235:957–972
Curthoys IS, MacDougall HG, Vidal PP, de Waele C (2017) Sustained and transient vestibular systems: a physiological basis for interpreting vestibular function. Front Neurol 8:117
Curthoys IS, Grant JW, Burgess AM et al (2018) Otolithic receptor mechanisms for vestibular-evoked myogenic potentials: a review. Front Neurol 9:366
Curthoys IS, Burgess AM, Goonetilleke SC (2019) Phase-locking of irregular guinea pig primary vestibular afferents to high frequency (〉250 Hz) sound and vibration. Hear Res 373:59–70
Curthoys IS, Grant W, Pastras CJ (2019) A review of mechanical and synaptic processes in otolith transduction of sound and vibration for clinical VEMP testing. J Neurophysiol 122:259–276
de Burlet HM (1924) Zur Innervation der Macula sacculi bei Säugetieren. Anat Anzeig 58:26–32
Dlugaiczyk J (2017) Ocular vestibular evoked myogenic potentials: where are we now? Otol Neurotol 38:e513–e521
Dlugaiczyk J (2019) Functional aspects of vestibular evoked myogenic potentials. In: Fritzsch B, Straka H et al (eds) The senses: a comprehensive reference, 2 edn. Elsevier, Amsterdam
Dlugaiczyk J, Burgess AM, Goonetilleke SC, Sokolic L, Curthoys IS (2019) Superior canal dehiscence syndrome: relating clinical findings with vestibular neural responses from a guinea pig model. Otol Neurotol 40:e406–e414
Dlugaiczyk J, Gensberger KD, Straka H (2019) Galvanic vestibular stimulation: from basic concepts to clinical applications. J Neurophysiol 121:2237–2255
Eatock RA, Songer JE (2011) Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34:501–534
Fujimoto C, Suzuki S, Kinoshita M et al (2018) Clinical features of otolith organ-specific vestibular dysfunction. Clin Neurophysiol 129:238–245
Goldberg JM (2000) Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130:277–297
Goto F, Meng H, Bai R et al (2004) Eye movements evoked by selective saccular nerve stimulation in cats. Auris Nasus Larynx 31:220–225
Govender S, Dennis DL, Colebatch JG (2015) Vestibular evoked myogenic potentials (VEMPs) evoked by air- and bone-conducted stimuli in vestibular neuritis. Clin Neurophysiol 126:2004–2013
Grant W, Curthoys I (2017) Otoliths—accelerometer and seismometer; implications in vestibular evoked myogenic potential (VEMP). Hear Res 353:26–35
Gürkov R, Jerin C, Flatz W, Maxwell R (2018) Dehiszenzsyndrom des oberen Bogenganges. Diagnostik mit vestibulär evozierten myogenen Potentialen und Fremitusnystagmus. HNO 66:390–395
Halmagyi GM, Chen L, MacDougall HG et al (2017) The video head impulse test. Front Neurol 8:258
Hecker DJ, Lohscheller J, Schorn B et al (2014) Electromotive triggering and single sweep analysis of vestibular evoked myogenic potentials (VEMPs). IEEE Trans Neural Syst Rehabil Eng 22:158–167
Interacoustics (2015) Technical specifications eclipse, p 8
International Standard ISO 389-6:2007(E): Acoustics—Reference zero for the calibration of audiometric equipment. Part 6: Reference threshold of hearing for test signals of short duration. First edition: 2007-07-01, reviewed and confirmed in 2015.
Isu N, Graf W, Sato H et al (2000) Sacculo-ocular reflex connectivity in cats. Exp Brain Res 131:262–268
Iwasaki S, McGarvie LA, Halmagyi GM et al (2007) Head taps evoke a crossed vestibulo-ocular reflex. Neurology 68:1227–1229
Iwasaki S, Smulders YE, Burgess AM et al (2008) Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol 119:2135–2147
Iwasaki S, Smulders YE, Burgess AM et al (2008) Ocular vestibular evoked myogenic potentials in response to bone-conducted vibration of the midline forehead at Fz. A new indicator of unilateral otolithic loss. Audiol Neurootol 13:396–404
Iwasaki S, Chihara Y, Smulders YE et al (2009) The role of the superior vestibular nerve in generating ocular vestibular-evoked myogenic potentials to bone conducted vibration at Fz. Clin Neurophysiol 120:588–593
Janky KL, Nguyen KD, Welgampola M et al (2013) Air-conducted oVEMPs provide the best separation between intact and superior canal dehiscent labyrinths. Otol Neurotol 34:127–134
Kantner C, Gürkov R (2012) Characteristics and clinical applications of ocular vestibular evoked myogenic potentials. Hear Res 294:55–63
Lee JD, Park MK, Lee BD et al (2011) Otolith function in patients with head trauma. Eur Arch Otorhinolaryngol 268:1427–1430
Lim LJ, Dennis DL, Govender S, Colebatch JG (2013) Differential effects of duration for ocular and cervical vestibular evoked myogenic potentials evoked by air- and bone-conducted stimuli. Exp Brain Res 224:437–445
MacDougall HG, Holden J, Rosengren SM, Chiarovano E (2018) μVEMP: a portable interface to record vestibular evoked myogenic potentials (VEMPs) with a smart phone or tablet. Front Neurol 9:543
Maheu M, Alvarado-Umanzor JM, Delcenserie A, Champoux F (2017) The clinical utility of vestibular-evoked myogenic potentials in the diagnosis of Ménière’s disease. Front Neurol 8:415
Manzari L, Tedesco A, Burgess AM, Curthoys IS (2010) Ocular vestibular-evoked myogenic potentials to bone-conducted vibration in superior vestibular neuritis show utricular function. Otolaryngol Head Neck Surg 143:274–280
Manzari L, Burgess AM, Curthoys IS (2012) Ocular and cervical vestibular evoked myogenic potentials in response to bone-conducted vibration in patients with probable inferior vestibular neuritis. J Laryngol Otol 126:683–691
Manzari L, Burgess AM, Curthoys IS (2012) Is it possible to measure peripheral vestibular function in a patient with congenital nystagmus? Eur Arch Otorhinolaryngol 269:349–352
Manzari L, Burgess AM, McGarvie LA et al (2012) Ocular and cervical vestibular evoked myogenic potentials to 500 Hz Fz bone-conducted vibration in superior semicircular canal dehiscence. Ear Hear 33:508–520
Manzari L, Burgess AM, McGarvie LA et al (2013) An indicator of probable semicircular canal dehiscence: ocular vestibular evoked myogenic potentials to high frequencies. Otolaryngol Head Neck Surg 149:142–145
Murofushi T, Nakahara H, Yoshimura E, Tsuda Y (2011) Association of air-conducted sound oVEMP findings with cVEMP and caloric test findings in patients with unilateral peripheral vestibular disorders. Acta Otolaryngol 131:945–950
Murofushi T (2016) Clinical application of vestibular evoked myogenic potential (VEMP). Auris Nasus Larynx 43:367–376
Nam JH, Grant JW, Rowe MH, Peterson EH (2019) Multi-scale modeling of mechanotransduction in the utricle. J Neurophysiol 122:132–150
Newlands SD, Vrabec JT, Purcell IM et al (2003) Central projections of the saccular and utricular nerves in macaques. J Comp Neurol 466:31–47
Nguyen KD, Welgampola MS, Carey JP (2010) Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol 31:793–802
Oh SY, Kim JS, Yang TH, Shin BS, Jeong SK (2013) Cervical and ocular vestibular-evoked myogenic potentials in vestibular neuritis: comparison between air- and bone-conducted stimulation. J Neurol 260:2102–2109
Oh SY, Kim HJ, Kim JS (2016) Vestibular-evoked myogenic potentials in central vestibular disorders. J Neurol 263:210–220
Papathanasiou ES, Murofushi T, Akin FW, Colebatch JG (2014) International guidelines for the clinical application of cervical vestibular evoked myogenic potentials: an expert consensus report. Clin Neurophysiol 125:658–666
Papathanasiou ES (2015) The evidence is finally here: ocular vestibular evoked myogenic potentials are mainly dependent on utricular pathway function. Clin Neurophysiol 126:1843–1844
Pastras CJ, Curthoys IS, Brown DJ (2017) In vivo recording of the vestibular microphonic in mammals. Hear Res 354:38–47
Pastras CJ, Curthoys IS, Brown DJ (2018) Dynamic response to sound and vibration of the guinea pig utricular macula, measured in vivo using Laser Doppler Vibrometry. Hear Res 370:232–237
Piker EG, Jacobson GP, Burkard RF et al (2013) Effects of age on the tuning of the cVEMP and oVEMP. Ear Hear 34:e65–e73
Portnuff CDF, Kleindienst S, Bogle JM (2017) Safe use of acoustic vestibular-evoked myogenic potential stimuli: protocol and patient-specific considerations. J Am Acad Audiol 28:708–717
Purves D, Augustine GJ, Fitzpatrick D (2001) The vestibular system. In: Purves D, Augustine GJ, Fitzpatrick D (eds) Neuroscience, 2 edn. Sinauer Associates, Sunderland, pp 297–316
Rahne T, Plößl S, Plontke SK, Strauss C (2017) Präoperative Bestimmung des Ursprungsnervs von Vestibularisschwannomen. HNO 65:966–972
Rosengren SM, McAngus TNP, Colebatch JG (2005) Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol 116:1938–1948
Rosengren SM, Govender S, Colebatch JG (2011) Ocular and cervical vestibular evoked myogenic potentials produced by air- and bone-conducted stimuli: comparative properties and effects of age. Clin Neurophysiol 122:2282–2289
Rosengren SM, Colebatch JG, Straumann D, Weber KP (2013) Why do oVEMPs become larger when you look up? Explaining the effect of gaze elevation on the ocular vestibular evoked myogenic potential. Clin Neurophysiol 124:7857–7891
Rosengren SM, Colebatch JG, Young AS, Govender S, Welgampola MS (2019) Vestibular evoked myogenic potentials in practice: methods, pitfalls and clinical applications. Clin Neurophysiol Pract 4:47–68
Rosowski JJ, Songer JE, Nakajima HH et al (2004) Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol 25:323–332
Songer JE, Eatock RA (2013) Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33:3706–3724
Suzuki JI, Tokumasu K, Goto K (1969) Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol 68:350–362
Uchino Y, Sasaki M, Sato H, Bai R, Kawamoto E (2005) Otolith and canal integration on single vestibular neurons in cats. Exp Brain Res 164:271–285
Uchino Y, Kushiro K (2011) Differences between otolith- and semicircular canal-activated neural circuitry in the vestibular system. Neurosci Res 71:315–327
Verrecchia L, Brantberg K, Tawfique Z, Maoli D (2019) Diagnostic accuracy of ocular vestibular evoked myogenic potentials for superior canal dehiscence syndrome in a large cohort of dizzy patients. Ear Hear 40:287–294
Walther LE, Hörmann K, Pfaar O (2010) Die Ableitung zervikaler und okulärer vestibular evozierter myogener Potentiale. Teil 2: Einflussfaktoren, Bewertung der Befunde und klinische Bedeutung. HNO 58:1129–1142
Walther LE, Repik I (2012) Neuritis des N. vestibularis inferior. Diagnose mittels VEMP-Diagnostik. HNO 60:126–131
Ward BK, Carey JP, Minor LB (2017) Superior canal dehiscence syndrome: lessons from the first 20 years. Front Neurol 8:177
Weber KP, Rosengren SM (2015) Clinical utility of ocular vestibular-evoked myogenic potentials (oVEMPs). Curr Neurol Neurosci Rep 15:22
Welgampola MS, Colebatch JG (2001) Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol 112:1971–1979
Welgampola MS, Colebatch JG (2005) Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology 64:1682–1688
Welgampola MS, Myrie OA, Minor LB et al (2008) Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology 70:464–472
Zuniga MG, Janky KL, Nguyen KD, Welgampola MS, Carey JP (2013) Ocular versus cervical VEMPs in the diagnosis of superior semicircular canal dehiscence syndrome. Otol Neurotol 34:121–126
Acknowledgements
I am grateful to the two anonymous reviewers for their comments and ideas. Furthermore, I would like to thank Prof. Marianne Dieterich (Munich), Prof. Ian Curthoys (Sydney) and Laura Fröhlich, M.Sc. (Halle/Saale) for a critical reading of the manuscript and helpful discussions.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Dlugaiczyk received reimbursement of travel and congress fees by Hennig and Otometrics, speaker honoraria by Otometrics and research grants by Otonomy outside this study.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case. Additional written informed consent was obtained from all individual participants or their legal representatives for whom identifying information is included in this article.
The supplement containing this article is not sponsored by industry.
Caption Electronic Supplementary Material
106_2019_767_MOESM1_ESM.pdf
Further information on the following topics: S1: The striola: an ideal “jerk” detector. S2: Sound and vibration as otolithic stimuli. S3: Vestibular microphonics. S4: Superior canal dehiscence
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dlugaiczyk, J. Evidence-based diagnostic use of VEMPs. HNO 68 (Suppl 2), 69–78 (2020). https://doi.org/10.1007/s00106-019-00767-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00106-019-00767-2