Abstract
Background
Crush injury/crush syndrome (CI/CS) is the second most common cause of death during earthquakes. Most studies of CI/CS have mainly focused on kidney injury after decompression. Few studies have focused on myocardial injury caused by crush injury and its potential mechanisms.
Methods
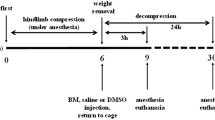
We first verified cardiomyocyte injury during compression in rats with a crush injury. The survival rate, electrocardiographic results, histological results, catecholamine changes and cardiac β1-AR expression were evaluated. Next, we explored the effects of pretreatment with a selective β1-blocker (bisoprolol) with or without fluid resuscitation on rats with a crush injury. In addition to evaluating the survival rates, biochemical and histological analyses and echocardiographic measurements were also performed.
Results
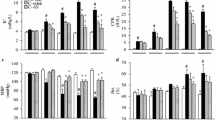
Reduced heart rates, elevated ST segments, and tall-peaked T waves were observed in the rats with a crush injury. The changes in the myocardial enzymes and pathological results demonstrated that myocardial damage occurred during compression in rats with a crush injury. The levels of the catecholamine norepinephrine in both the serum and myocardial tissue were elevated during compression. Pretreatment with a selective β1-blocker combined with fluid resuscitation significantly improved the survival rates of the rats with lethal crush injury. The myocardial enzymes and pathological results showed that the combined therapy decreased myocardial damage. The echocardiography measurements showed that the rats that received the combined therapy exhibited decreased left ventricular mass (LVM), left ventricular volume at end-systole (LVVs) and left ventricular internal diameter (LVID) compared with the rats with a crush injury.
Conclusions
Our findings demonstrated the presence of myocardial injury in the early stage of compression in rats with a crush injury. Pretreatment with a β1-blocker (bisoprolol) with fluid resuscitation significantly reduced mortality, decreased myocardial tissue damage, and improved ventricular remodelling in rats with a lethal crush injury.





Similar content being viewed by others
References
Speck K, Schneider BS, Deashinta N. A rodent model to advance the field treatment of crush muscle injury during earthquakes and other natural disasters. Biol Res Nurs. 2013;15(1):17–25. https://doi.org/10.1177/1099800411414698.
Sever MS, Vanholder R. Crush syndrome: a case report and review of the literature. J Emerg Med. 2015;48(6):730–1. https://doi.org/10.1016/j.jemermed.2014.07.063.
Sever MS, Vanholder R. Management of crush syndrome casualties after disasters. Rambam Maimonides Med J. 2011;2(2):e0039. https://doi.org/10.5041/RMMJ.10039.
Zhang BF, Wang PF, Cong YX, Lei JL, Wang H, Huang H, et al. Anti-high mobility group box-1 (HMGB1) antibody attenuates kidney damage following experimental crush injury and the possible role of the tumor necrosis factor-alpha and c-Jun N-terminal kinase pathway. J Orthop Surg Res. 2017;12(1):110. https://doi.org/10.1186/s13018-017-0614-z.
Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20(1):135. https://doi.org/10.1186/s13054-016-1314-5.
Zhang L, Fu P, Wang L, Cai G, Zhang L, Chen D, et al. The clinical features and outcome of crush patients with acute kidney injury after the Wenchuan earthquake: differences between elderly and younger adults. Injury. 2012;43(9):1470–5. https://doi.org/10.1016/j.injury.2010.11.036.
Guo X, Wang D, Liu Z. Electrocardiographic changes after injury in a rat model of combined crush injury. Am J Emerg Med. 2013;31(12):1661–5. https://doi.org/10.1016/j.ajem.2013.08.054.
Liu S, Yu Y, Luo B, Liao X, Tan Z. Impact of traumatic muscle crush injury as a cause of cardiomyocyte-specific injury: an experimental study. Heart Lung Circ. 2013;22(4):284–90. https://doi.org/10.1016/j.hlc.2012.11.008.
Rajagopalan S. Crush injuries and the crush syndrome. Med J Armed Forces India. 2010;66(4):317–20. https://doi.org/10.1016/S0377-1237(10)80007-3.
Loftus TJ, Efron PA, Moldawer LL, Mohr AM. Beta-blockade use for traumatic injuries and immunomodulation: a review of proposed mechanisms and clinical evidence. Shock. 2016;46(4):341–51. https://doi.org/10.1097/SHK.0000000000000636.
Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113(6):739–53. https://doi.org/10.1161/CIRCRESAHA.113.300308.
Samson R, Baydoun H, Jaiswal A, Le Jemtel TH. Cardiac adrenergic nervous system and left ventricular remodeling. Am J Med Sci. 2015;350(4):321–6. https://doi.org/10.1097/MAJ.0000000000000549.
Morey TE, Modell JH, Shekhawat D, Grand T, Shah DO, Gravenstein N, et al. Preparation and anesthetic properties of propofol microemulsions in rats. Anesthesiology. 2006;104(6):1184–90. https://doi.org/10.1097/00000542-200606000-00013.
Hu C, Flecknell PA, Liles JH. Fentanyl and medetomidine anaesthesia in the rat and its reversal using atipamazole and either nalbuphine or butorphanol. Lab Anim. 1992;26(1):15–22. https://doi.org/10.1258/002367792780809075.
Nakayama T, Fujita M, Ishihara M, Ishihara M, Ogata S, Yamamoto Y, et al. Improved survival rate by temperature control at compression sites in rat model of crush syndrome. J Surg Res. 2014;188(1):250–9. https://doi.org/10.1016/j.jss.2013.12.012.
Huang J, Ma WZ. Studies on mechanism of myocardial injury induced by adrenergic alpha receptors and catecholamines. Chin J Pathophysiol. 1990;6(5):313–6.
Debelle FD, Nortier JL, De Prez EG, Garbar CH, Vienne AR, Salmon IJ, et al. Aristolochic acids induce chronic renal failure with interstitial fibrosis in salt-depleted rats. J Am Soc Nephrol. 2002;13(2):431–6.
Smith KM, Mrozek JD, Simonton SC, Bing DR, Meyers PA, Connett JE, et al. Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit Care Med. 1997;25(11):1888–97.
Takikawa M, Nakamura S, Ishihara M, Takabayashi Y, Fujita M, Hattori H, et al. Improved angiogenesis and healing in crush syndrome by fibroblast growth factor-2-containing low-molecular-weight heparin (Fragmin)/protamine nanoparticles. J Surg Res. 2015;196(2):247–57. https://doi.org/10.1016/j.jss.2015.03.022.
Stypmann J, Engelen MA, Troatz C, Rothenburger M, Eckardt L, Tiemann K. Echocardiographic assessment of global left ventricular function in mice. Lab Anim. 2009;43(2):127–37. https://doi.org/10.1258/la.2007.06001e.
Aoki T, Takahashi J, Fukumoto Y, Yasuda S, Ito K, Miyata S, et al. Effect of the Great East Japan Earthquake on cardiovascular diseases—report from the 10 hospitals in the disaster area. Circ J. 2013;77(2):490–3. https://doi.org/10.1253/circj.cj-12-1594.
Adachi K, Kawata M, Araki S, Matsumoto A, Mukai T, Ikoma T. A case of crush syndrome with giant negative T waves and reversible left ventricular dysfunction. Jpn Circ J. 1996;60(10):809–14.
Allister C. Cardiac arrest after crush injury. Br Med J (Clin Res Ed). 1983;287(6391):531–2. https://doi.org/10.1136/bmj.287.6391.531-a.
Better OS, Abassi Z, Rubinstein I, Marom S, Winaver Y, Silberman M. The mechanism of muscle injury in the crush syndrome: ischemic versus pressure-stretch myopathy. Miner Electrolyte Metab. 1990;16(4):181.
Szardien S, Mollmann H, Willmer M, Akashi YJ, Hamm CW, Nef HM. Mechanisms of stress (takotsubo) cardiomyopathy. Heart Fail Clin. 2013;9(2):197–205, ix. https://doi.org/10.1016/j.hfc.2012.12.012.
Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118(4):397–409. https://doi.org/10.1161/CIRCULATIONAHA.106.677625.
Han X. Restraint stress aggravates rat heart injury caused by a crush injury through endoplasmic reticulum stress. Shijiazhuang: Hebei Medical University; 2013.
Geng J, Zhang XJ, Ma CL, Li YM, Zhang GZ, Ma RF, et al. Restraint stress aggravates rat kidney injury caused by a crush injury through endoplasmic reticulum stress. J Trauma Acute Care Surg. 2013;75(5):798–806. https://doi.org/10.1097/TA.0b013e3182a685ff.
Myslivecek J, Ricny J, Palkovits M, Kvetnansky R. The effects of short-term immobilization stress on muscarinic receptors, beta-adrenoceptors, and adenylyl cyclase in different heart regions. Ann N Y Acad Sci. 2004;1018:315–22. https://doi.org/10.1196/annals.1296.038.
Qi Y. Cardiac sympathetic nerve norepinephrine transporter and β-adrenergic receptor internalization of rat under immobilization. Shijiazhuang: Hebei Medical University; 2016.
Bondarenko VE. A compartmentalized mathematical model of the beta1-adrenergic signaling system in mouse ventricular myocytes. PLoS ONE. 2014;9(2):e89113. https://doi.org/10.1371/journal.pone.0089113.
Tillinger A, Novakova M, Krizanova O, Kvetnansky R, Myslivecek J. Heart ventricles specific stress-induced changes in beta-adrenoceptors and muscarinic receptors. Gen Physiol Biophys. 2014;33(3):357–64. https://doi.org/10.4149/gpb_2014002.
Use ABT. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). CIBIS Investigators and Committees. Circulation. 1994;90(4):1765–73.
Ueyama T, Kasamatsu K, Hano T, Yamamoto K, Tsuruo Y, Nishio I. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of ‘tako-tsubo’ cardiomyopathy. Circ J. 2002;66(7):712–3.
Murata I, Ooi K, Shoji S, Motohashi Y, Kan M, Ohtake K, et al. Acute lethal crush-injured rats can be successfully rescued by a single injection of high-dose dexamethasone through a pathway involving PI3K-Akt-eNOS signaling. J Trauma Acute Care Surg. 2013;75(2):241–9. https://doi.org/10.1097/TA.0b013e3182905f11.
Kobayashi J, Murata I. Nitrite as a pharmacological intervention for the successful treatment of crush syndrome. Physiol Rep. 2018. https://doi.org/10.14814/phy2.13633.
Murata I, Nozaki R, Ooi K, Ohtake K, Kimura S, Ueda H, et al. Nitrite reduces ischemia/reperfusion-induced muscle damage and improves survival rates in rat crush injury model. J Trauma Acute Care Surg. 2012;72(6):1548–54. https://doi.org/10.1097/TA.0b013e31824a76b5.
Cuong NT, Abe C, Binh NH, Hara A, Morita H, Ogura S. Sivelestat improves outcome of crush injury by inhibiting high-mobility group box 1 in rats. Shock. 2013;39(1):89–95. https://doi.org/10.1097/SHK.0b013e31827a2412.
Shimazaki J, Matsumoto N, Ogura H, Muroya T, Kuwagata Y, Nakagawa J, et al. Systemic involvement of high-mobility group box 1 protein and therapeutic effect of anti-high-mobility group box 1 protein antibody in a rat model of crush injury. Shock. 2012;37(6):634–8. https://doi.org/10.1097/SHK.0b013e31824ed6b7.
Garcha AS, Cohen DL. Catecholamine excess: pseudopheochromocytoma and beyond. Adv Chronic Kidney Dis. 2015;22(3):218–23.
Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383(9932):1933–43. https://doi.org/10.1016/S0140-6736(14)60107-0.
Herum KM, Lunde IG, McCulloch AD, Christensen G. The soft- and hard-heartedness of cardiac fibroblasts: mechanotransduction signaling pathways in fibrosis of the heart. J Clin Med. 2017. https://doi.org/10.3390/jcm6050053.
Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–74. https://doi.org/10.1007/s00018-013-1349-6.
Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87(4):1285–342. https://doi.org/10.1152/physrev.00012.2007.
Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128(4):388–400. https://doi.org/10.1161/CIRCULATIONAHA.113.001878.
Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114(1):32–9. https://doi.org/10.1161/CIRCULATIONAHA.106.613414.
Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58(12):1271–9.
Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57(7):821–8. https://doi.org/10.1016/j.jacc.2010.06.062.
Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182(5):652–60.
Prabhu SD, Chandrasekar B, Murray DR, Freeman GL. Beta-adrenergic blockade in developing heart failure: effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation. 2000;101(17):2103–9.
Bonnefont-Rousselot D, Mahmoudi A, Mougenot N, Varoquaux O, Le Nahour G, Fouret P, et al. Catecholamine effects on cardiac remodelling, oxidative stress and fibrosis in experimental heart failure. Redox Rep. 2002;7(3):145–51. https://doi.org/10.1179/135100002125000389.
Watanabe K, Ohta Y, Inoue M, Ma M, Wahed MI, Nakazawa M, et al. Bisoprolol improves survival in rats with heart failure. J Cardiovasc Pharmacol. 2001;38(Suppl 1):S55–8.
Kadioglu E, Teksen Y, Kocak C, Kocak FE. Beneficial effects of bardoxolone methyl, an Nrf2 activator, on crush-related acute kidney injury in rats. Eur J Trauma Emerg Surg. 2019. https://doi.org/10.1007/s00068-019-01216-z.
Nishikata R, Kato N, Hiraiwa K. Oxidative stress may be involved in distant organ failure in tourniquet shock model mice. Leg Med (Tokyo). 2014;16(2):70–5. https://doi.org/10.1016/j.legalmed.2013.11.004.
Matsumoto H, Matsumoto N, Shimazaki J, Nakagawa J, Imamura Y, Yamakawa K, et al. Therapeutic effectiveness of anti-RAGE antibody administration in a rat model of crush injury. Sci Rep. 2017;7(1):12255. https://doi.org/10.1038/s41598-017-12065-4.
Acknowledgements
The authors thank the personnel of the Institute of Disaster Medicine of Tianjin University for support and assistance.
Funding
This work was supported by National Key Research and Development Program (2018YFC1504402, 2016YFC0802806), Tianjin University Independent Innovation Fund (2019XZS-0021).
Author information
Authors and Affiliations
Author notes
Prof. Shike Hou takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yu, M., Lv, Q., Shi, J. et al. β1-Blocker improves survival and ventricular remodelling in rats with lethal crush injury. Eur J Trauma Emerg Surg 48, 455–470 (2022). https://doi.org/10.1007/s00068-020-01408-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-020-01408-y