Abstract
Purpose
We investigated whether radiologic parameters by dynamic contrast-enhanced (DCE) integrated magnetic resonance–positron-emission tomography (MR-PET) predicts tumor response to treatment and survival in non-metastatic non-small-cell lung cancer (NSCLC) patients receiving chemoradiotherapy (CRT).
Methods
Patients underwent DCE integrated MR-PET imaging 1 week before CRT. The following parameters were analyzed: primary tumor size, gross tumor volume, maximal standardized uptake value (SUVmax), total lesion glycolysis (TLG), apparent diffusion coefficient (ADC), volume transfer constant (Ktrans), reverse reflux rate constant (kep), extracellular extravascular volume fraction (ve), blood plasma volume fraction (vp), and initial area under the time-concentration curve defined over the first 60 s post-enhancement (iAUC60). CRT responses were defined using the revised Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1).
Results
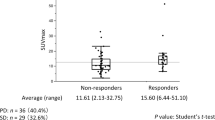
Thirty patients were included. Non-responders demonstrated higher baseline TLG (p = 0.012), and lower baseline Ktrans (p = 0.020) and iAUC60 (p = 0.016) compared to responders, indicating the usefulness of DCE integrated MR-PET to predict treatment responses. Receiver operating characteristic curve indicated that TLG has the best differentiation capability to predict responders. By setting the threshold of TLG to 277, the sensitivity, specificity, and accuracy were 66.7%, 83.3%, and 75.0%, respectively, with an area under the curve of 0.776. The median follow-up time was 19.6 (range 7.8–32.0) months. In univariate analyses, baseline TLG >277 (p = 0.005) and baseline Ktrans <254 (10−3 min−1; p = 0.015) correlated with poor survival after CRT. In multivariate analysis, baseline TLG >277 remained the significant factor in predicting progression (p = 0.012) and death (p = 0.031).
Conclusions
The radiologic parameters derived from DCE integrated MR-PET scans are useful for predicting treatment response in NSCLC patients treated with CRT; furthermore, these parameters are correlated with clinical and survival outcomes including tumor progression and death.
Zusammenfassung
Ziel
Wir untersuchten, ob radiologische Parameter durch die dynamische kontrastverstärkte (DCE) integrierte Magnetresonanz-Positronenemissionstomographie (MR-PET) das Ansprechen des Tumors auf die Behandlung und das klinische Überleben bei Patienten mit nichtmetastasiertem nicht-kleinzelligem Lungenkrebs (NSCLC), die eine primäre Chemoradiotherapie (CRT) erhalten haben, vorhersagen können.
Methoden
Die Patienten unterzogen sich 1 Woche vor CRT einer DCE-integrierten 3T-MR-PET-Bildgebung. Folgende Parameter wurden analysiert: Primärtumorgröße, Gesamttumorvolumen, maximaler standardisierter Aufnahmewert (SUVmax), Gesamtläsionsglykolyse (TLG), scheinbarer Diffusionskoeffizient (ADC), Volumentransfer konstant (Ktrans), Rückflussrate konstant (kep), extrazelluläre extravaskuläre Volumenfraktion (ve), Blutplasma-Volumenfraktion (vp), und Anfangsbereich unter der Zeit-Konzentrationskurve, die über die ersten 60s nach der Verbesserung definiert wurde (iAUC60). Die CRT-Antworten wurden anhand der überarbeitete Response-Bewertungskriterien bei soliden Tumoren(RECIST)-Richtlinie (Version 1.1) definiert.
Ergebnisse
Dreißig Patienten wurden aufgenommen. Non-Responder zeigten im Vergleich zu den Respondern eine höhere Baseline-TLG (p = 0,012), eine niedrigere Baseline Ktrans (p = 0,020) und iAUC60 (p = 0,016), was auf den Nutzen von DCE-integriertem MR-PET für die Vorhersage von Behandlungsreaktionen hinweist. Die „Receiver-operating-characteristic“-(ROC-)Kurve zeigte, dass TLG die beste Unterscheidungsfähigkeit zur Vorhersage von Respondern hat. Durch die Festlegung des Schwellenwerts des TLG auf 277 betrugen Sensitivität, Spezifität und Genauigkeit jeweils 66,7, 83,3 und 75,0%, mit einer Fläche unter der Kurve von 0,776. Das durchschnittliche Follow-up betrug 19,6 Monate (Spanne 7,8–32,0 Monate). In univariaten Analysen korrelierten Baseline TLG >277 (p = 0,005) und Baseline Ktrans <254 (10−3 min−1; p = 0,015) mit einer schlechten Überlebenschance nach CRT. In der multivariaten Analyse blieb die Baseline-TLG >277 der signifikante Faktor bei der Prognose von Progression (p = 0,012) und Tod (p = 0,031).
Schlussfolgerung
Die radiologischen Parameter, die von DCE-integrierten MR-PET-Scans abgeleitet werden, sind nützlich für die Vorhersage des Behandlungserfolgs bei NSCLC-Patienten, die mit CRT behandelt werden, und sind mit der klinischen Überlebensrate einschließlich Tumorprogression und Tod korreliert.



Similar content being viewed by others
References
Ramnath N, Dilling TJ, Harris LJ et al (2013) Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 143:e314S–e340S
Postmus PE, Kerr KM, Oudkerk M et al (2017) Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv1–iv21
O’Rourke N, Roque IFM, Farre Bernado N et al (2010) Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002140.pub3
Warner A, Dahele M, Hu B et al (2016) Factors associated with early mortality in patients treated with concurrent chemoradiation therapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 94:612–620
Huang YS, Chen JL, Hsu FM et al (2018) Response assessment of stereotactic body radiation therapy using dynamic contrast-enhanced integrated MR-PET in non-small cell lung cancer patients. J Magn Reson Imaging 47:9
Ohno Y, Koyama H, Yoshikawa T et al (2012) Diffusion-weighted MRI versus 18F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol 198:75–82
Chang YC, Yu CJ, Chen CM et al (2012) Dynamic contrast-enhanced MRI in advanced nonsmall-cell lung cancer patients treated with first-line bevacizumab, gemcitabine, and cisplatin. J Magn Reson Imaging 36:387–396
Tao X, Wang L, Hui Z et al (2016) DCE-MRI perfusion and permeability parameters as predictors of tumor response to CCRT in Patients with locally advanced NSCLC. Sci Rep 6:35569
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Yabuuchi H, Hatakenaka M, Takayama K et al (2011) Non-small cell lung cancer: detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology 261:598–604
Jeong JU, Chung WK, Nam TK et al (2014) Early metabolic response on 18F-fluorodeoxyglucose-positron-emission tomography/computed tomography after concurrent chemoradiotherapy for advanced stage III non-small cell lung cancer is correlated with local tumor control and survival. Anticancer Res 34:2517–2523
Chen HH, Chiu NT, Su WC et al (2012) Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology 264:559–566
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Sobin LH, Gospodarowicz MK, Wittekind C (2009) TNM Classification of Malignant Tumours, 7th edn. Wiley-Blackwell, New Jersey
Surasi DS, Bhambhvani P, Baldwin JA et al (2014) (1)(8)F-FDG PET and PET/CT patient preparation: a review of the literature. J Nucl Med Technol 42:5–13
Chen JL, Huang YS, Kuo SH et al (2013) Intensity-modulated radiation therapy for T4 nasopharyngeal carcinoma. Treatment results and locoregional recurrence. Strahlenther Onkol 189:1001–1008
Auperin A, Le Pechoux C, Pignon JP et al (2006) Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 17:473–483
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341–1346
Huang YS, Hsu HH, Chen JY et al (2014) Quantitative computed tomography of pulmonary emphysema and ventricular function in chronic obstructive pulmonary disease patients with pulmonary hypertension. Korean J Radiol 15:871–877
Blumenthal GM, Karuri SW, Zhang H et al (2015) Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol 33:1008–1014
Bradley JD, Paulus R, Komaki R et al (2015) Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16:187–199
Wahl RL, Jacene H, Kasamon Y et al (2009) From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122s–150s
Ohno Y, Fujisawa Y, Koyama H et al (2017) Dynamic contrast-enhanced perfusion area-detector CT assessed with various mathematical models: Its capability for therapeutic outcome prediction for non-small cell lung cancer patients with chemoradiotherapy as compared with that of FDG-PET/CT. Eur J Radiol 86:83–91
Chen YF, Yuan A, Cho KH et al (2017) Functional evaluation of therapeutic response of HCC827 lung cancer to bevacizumab and erlotinib targeted therapy using dynamic contrast-enhanced and diffusion-weighted MRI. PLoS ONE 12:e187824
Vaupel P, Kelleher DK, Hockel M (2001) Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol 28:29–35
Chen JL, Chen JP, Huang YS et al (2016) Radiosensitization in esophageal squamous cell carcinoma: Effect of polo-like kinase 1 inhibition. Strahlenther Onkol 192:260–268
Antonia SJ, Villegas A, Daniel D et al (2017) Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919–1929
Acknowledgements
We thank Prof. Jin-Yuan Shih, Prof. Chao-Chi Ho, Dr. Tzu-Hsiu Tsai, Dr. Chia-Lin Hsu, Dr. Ching-Yao Yang, Dr. Chung-Yu Chen, and Dr. Sheng-Kai Liang for their guidance in conducting this work. We are thankful to the doctors, nurses, healthcare providers, and other sources of health information who contributed to this study. We also thank the staff of the Core Labs, Department of Medical Research, National Taiwan University Hospital, for their technical support.
Funding
This work was supported by the National Taiwan University Hospital (grant number: NTUH106-N3662 and NTUH107-M4007) and the Ministry of Science and Technology (MST, Taiwan, Contract numbers: MST 100-2221-E-002-031-MY3, 104-2221-E-002-093-MY3, and 107-2314-B-002-225-MY2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y.-S. Huang, J.L.-Y. Chen, J.-Y. Chen, Y.-F. Lee, J.-Y. Huang, S.-H. Kuo, R.-F. Yen and, Y.-C. Chang declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the National Taiwan University Hospital Research Ethics Committee (Approval number: 201501073RINA) and is registered with ClinicalTrials.gov, number NCT NCT03053804.
Rights and permissions
About this article
Cite this article
Huang, YS., Chen, J.LY., Chen, JY. et al. Predicting tumor responses and patient survival in chemoradiotherapy-treated patients with non-small-cell lung cancer using dynamic contrast-enhanced integrated magnetic resonance–positron-emission tomography. Strahlenther Onkol 195, 707–718 (2019). https://doi.org/10.1007/s00066-018-1418-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1418-8