Abstract
Since December 2019, a novel coronavirus (severe acute respiratory syndrome—coronavirus 2, SARS-CoV-2) has rapidly spread around the world resulting in an acute respiratory illness pandemic. The majority of patients presents with mild symptoms of coronavirus disease 2019 (COVID-19). However, about 5% become critically ill and require intensive care treatment. Acute hypoxemic failure with severe dyspnea and an increased respiratory rate (>30/min) usually leads to ICU admission. At that point, bilateral pulmonary infiltrates are typically seen. Patients often develop a severe acute respiratory distress syndrome (ARDS). To date there is no specific treatment available—the main goal of supportive therapy is to ascertain adequate oxygenation. Early intubation and repeated prone positioning are key elements in treating hypoxemic COVID-19 patients. Strict adherence to basic infection control measures (including hand hygiene) and use of personal protection equipment (PPE) are essential in the care of patients. Procedures that lead to formation of aerosols should be avoided where possible and carried out with utmost precaution.
Zusammenfassung
Seit Dezember 2019 verbreitet sich das neuartige Coronavirus SARS-CoV‑2 (Severe Acute Respiratory Syndrome – Corona Virus-2) rasch im Sinne einer weltweiten Pandemie. Dies stellt Kliniker und Krankenhäuser vor große Herausforderungen und belastet die Gesundheitssysteme vieler Länder in einem nie dagewesenen Maß.
Die Mehrheit der Patienten zeigt lediglich milde Symptome der sogenannten Coronavirus Disease 2019 (COVID-19). Dennoch benötigen etwa 5 % eine intensivmedizinische Behandlung. Die akute hypoxämische respiratorische Insuffizienz mit Dyspnoe und hoher Atemfrequenz (>30/min) führt in der Regel zur Aufnahme auf die Intensivstation. Oft finden sich dann bereits bilaterale pulmonale Infiltrate in der Bildgebung und im Verlauf entwickeln viele Patienten ein Acute Respiratory Distress Syndrome (ARDS).
Bisher gibt es noch keine zugelassene spezifische Therapieoption. Das Hauptziel der supportiven Therapie ist, eine ausreichende Oxygenierung sicherzustellen. Die frühe Intubation und wiederholte Bauchlagerung sind dabei wichtige Elemente in der Behandlung von hypoxämischen COVID-19 Patienten.
Die strikte Einhaltung der Basishygiene, einschließlich der Händehygiene, sowie das korrekte Tragen von adäquater persönlicher Schutzausrüstung ist im Umgang mit den Patienten unabdingbar. Prozeduren, die zur Aerosolbildung führen könnten, sollten, soweit möglich, vermieden, und, falls nötig, mit äußerster Sorgfalt und Vorbereitung durchgeführt werden.
Similar content being viewed by others
Remarks
Since December 2019, a novel coronavirus (severe acute respiratory syndrome—coronavirus 2, SARS-CoV-2) has rapidly been transmitted around the world resulting in an acute respiratory illness pandemic. The clinical picture, coronavirus disease 2019 (COVID-19), has affected thousands of people so far and created an unprecedented strain on many health care systems.
Background
These recommendations aim to give guidance to physicians treating COVID-19 patients on their ICUs. Experiences from China and Italy, where we see a high case load, currently serve as our benchmark, while acknowledging that the pandemic is still in an early, dynamic stage and that experience and scientific evidence will grow [1]. Comprehensive information on the pathogen and the trajectory of the pandemic is available online through the Robert Koch-Institut (RKI, www.rki.de).
We strongly recommend a multidisciplinary approach while tackling the challenges that ICUs and hospitals encounter with the management and treatment of COVID-19 patients. Aside from intensive care physicians and nurses, infectious disease and infection control specialists need to be part of the team.
Transmission of SARS-CoV‑2 usually occurs via droplet infection during close contact. Therefore, a strict implementation of basic infection control measures such as hand hygiene and the use of personal protection equipment (PPE) are essential.
Specimens and testing
The detection of SARS-CoV‑2 is carried out from a deep oropharyngeal swab or oropharyngeal lavage using real-time reverse transcriptase-polymerase chain reaction (RT-PCR). A patient with a negative test should be retested if there is a high clinical suspicion that they contracted the virus. The swab might become negative in a later stage of the disease (pneumonia, ARDS) while there is still infectious viral shedding in the lower airways. A PCR of endotracheal aspirates might be helpful in those cases.
Clinical disease pattern
Patients usually present with fever and dry cough as key features of COVID-19. In 81% of the patients the disease takes a mild course, 14% of the patients become severely ill, and, approximately 5% of the patients become critically ill [2]. Severe dyspnea with an increased work of breathing (respiratory rate >30/min) and hypoxemic respiratory failure typically lead to admission to ICU. At that stage, bilateral pulmonary infiltrates can often be seen on imaging [3]. Severely affected patients may develop ARDS or bacterial superinfection and septic shock. Further complications seen in those patients are myocardial dysfunction and arrhythmias as well as acute kidney failure. On average, it takes approximately 10 days from showing first symptoms to ICU admission [4].
Laboratory changes
In 80% of COVID-19 patients there is apparent lymphocytopenia, and, in one third of those patients this is accompanied by leukopenia. Most of the patients have normal levels of procalcitonin. However, a bacterial superinfection might trigger a significant increase. C‑Reactive protein is usually elevated, where very high values seem to correlate with worse outcomes [5]. Thrombocytopenia, and, an elevation of D‑dimers and lactate dehydrogenase (LDH) are found in approximately 40% of the patients. An increase in unspecific LDH, especially as high as or more than 400 IU/ml, seems to indicate a severe course of the disease. A small portion of patients also present with elevated troponin, of which the clinical implications are unknown.
Imaging
Conventional chest radiographs show bilateral infiltrates in COVID-19 patients treated on ICU. Even in early stages of the disease, computed tomography (CT) can reliably detect typical bilateral subpleural ground-glass opacities [6] and consolidation of the lungs [7]. However, due to the potential risks for health care workers and patients, we advise to only perform a CT in ICU patients when absolutely necessary for clinical decision making [8]. Bedside imaging, like ultrasonography, should be preferred.
Infection control
Patients should preferably be treated in isolation rooms ideally with a functional anteroom for donning and doffing PPE. As the epidemic/pandemic progresses, isolation of patients in cohorts is reasonable. Patients with COVID-19 should only be seen and cared for by trained personnel who do not have contact to other non-COVID-19 patients. The number of people working or visiting at bedside should be kept to a minimum—this also includes implementation of restrictions to visits by family and friends.
Personnel working at bedside must strictly adhere to basic infection control measures such as hand hygiene and consequently follow instructions on the use of personal protection equipment (PPE). According to the RKI, correct PPE consists of an impervious gown, gloves, tight-fitting facemask (FFP2 or FFP3 or respirator in case of strong exposure to aerosols, e.g., bronchoscopy) as well as goggles. It is important to frequently train health care workers on structured donning and removing of PPE, especially on tight mask-fitting and sequential hand disinfection. Comprehensive recommendations on infection control (rooms, protection of personnel, disinfection, cleaning, waste handling and patient transport) can be found online on the website of the RKI [9]. Local guidelines and standard procedures for hospitals should be implemented by a multidisciplinary expert panel.
Drugs
To date there is not enough data to recommend a specific antiviral treatment for COVID-19. Several drugs (hydroxychloroquine/chloroquine, lopinavir/ritonavir, camostat mesilate, remdesivir and others) have been considered as treatment options. We recommend to only use those options as part of compassionate use programs or approved study protocols after carefully evaluating risks and benefits for the individual patient [10]. The University of Liverpool has published possible pharmacokinetic interactions of experimental COVID-19 therapies that might support decision making [11].
Steroids should not routinely be used in ARDS patients as they seem to reduce viral clearance and promote opportunistic infections, e.g., fungal infections [12]. Studies in SARS and influenza showed a disadvantage for patients treated with steroids. However, in septic shock refractory to fluids and vasopressors, low-dose hydrocortisone is still indicated [13].
Antibiotics
As a principle, we recommend sampling of at least two blood cultures sets (aerobic and anaerobic at any one time) at the time of admission to ICU and whenever the patient worsens [13]. In patients suspected to have a bacterial superinfection an empiric broad-spectrum antibiotic therapy should be started as soon as possible. A prophylactic antibiotic treatment is not recommended.
Management of acute hypoxemic respiratory failure
Fluid management should be handled restrictively, especially in cases with no signs of shock or tissue malperfusion, as fluid overload further impairs oxygenation.
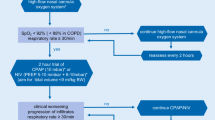
The goal is to ascertain adequate oxygenation. We recommend aiming for SpO2 ≥90% [14]. It is important to acknowledge that oxygen supplementation through high-flow nasal cannula (HFNC) and noninvasive ventilation (NIV) leads to aerosol formation. It is therefore absolutely necessary to make sure that HFNC and facemasks are fitted correctly to the patient [15], and, that the medical personnel at bedside strictly adheres to PPE instructions. Noninvasive ventilation with a helmet should be preferred where available.
In general, we advise to be rather restrictive with HFNC and NIV in the context of COVID-19. In patients with severe hypoxemia (PaO2/FiO2 ≤200 mm Hg) we suggest performing early intubation and invasive mechanical ventilation. In any case, continuous monitoring and preparedness for urgent intubation are cornerstones in the treatment of COVID-19 patients with respiratory failure. A delay of intubation in patients failing NIV worsens outcome, and, any emergency intubation in this cohort puts medical professionals at risk and should be avoided.
Intubation and other procedures
Airway procedures (intubation, bronchoscopy, open suction, bag ventilation, tracheostomy) should only be performed with appropriate airborne precautions PPE (including FFP2/FFP3 masks and goggles) if absolutely necessary due to the risk of aerosol formation. For invasive ventilation we recommend closed inline suction catheters. Any disconnection of the patient from the ventilator should be avoided to prevent lung decruitment and aerosolization. If necessary, the endotracheal tube should be clamped, and the ventilator disabled (to prevent aerosolization). Use of nebulizers is not recommended and use of metered dose inhalers are preferred where possible.
Intubation should be performed by the most experienced physician with a broad expertise in airway management. If possible, a rapid sequence induction without intermittent bag mask ventilation should be preferred to minimize aerosol formation [16]. With using a video-laryngoscope for intubation the distance between physician and patient during intubation can be increased. To verify correct tube placement avoid using the stethoscope and use direct visualization and CO2 indicators or capnography.
In case of a cardiopulmonary arrest and subsequent cardiopulmonary resuscitation the medical team has to make sure PPE is still used correctly. Airway management needs to be performed quickly and the number of people at the bedside should be kept to a minimum if possible.
Invasive ventilation and adjuvant treatments
In patients with ARDS, international guidelines recommend a tidal volume (TV) of ≤6 ml/kg ideal body weight and a plateau pressure of no more than 30 cm H2O. Positive end-expiratory pressure (PEEP) can be adjusted according to the ARDS network tables. Moderate and severe ARDS necessitates higher PEEP [17].
In ARDS with a PaO2/FiO2 <150 mm Hg prone positioning should be administered consistently for 16 h. If severe hypoxemia persists, prone positioning needs to be repeated.
As options for bridging in very severe cases inhaled NO, the administration of muscle relaxants and recruitment maneuvers may be considered.
Where available, in patients with severe ARDS and refractory hypoxemia (PaO2/FiO2 <80 or 60 mm Hg) veno-venous extracorporeal membrane oxygenation (ECMO) may serve as a therapeutic option to stabilize gas exchange. As it is a very complex and resource intensive treatment, all other measures should have been exhausted before considering ECMO, and thorough risk–benefit consideration, including presumed patient goals, is warranted.
Management of ICU capacity
The German ARDS-network and the working group Respiratory Failure within the German Interdisciplinary Association of Critical Care and Emergency Medicine (Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin) in cooperation with the RKI have launched a website that provides an overview of currently available ICU beds in Germany (https://www.intensivregister.de/#/intensivregister).
References
Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS (2020) Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. https://doi.org/10.1007/s00134-020-05979-7
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. https://doi.org/10.1001/jama.2020.2648
Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X et al (2020) CT imaging features of 2019 novel coronavirus (2019-ncoV). Radiology. https://doi.org/10.1148/radiol.2020200230
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H et al (2020) Clinical course and outcomes of critically ill patients with SARS-CoV‑2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(20)30079-5
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX et al (2020) Clinical characteristics of coronavirus disease 2019in China. N Engl J Med. https://doi.org/10.1056/NEJMoa2002032
Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L et al (2020) Chest CT findings in patients with corona virus disease 2019 and its relationship with clinical features. Invest Radiol. https://doi.org/10.1097/RLI.0000000000000670
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. https://doi.org/10.1016/S0140-6736(20)30183-5
Liao X, Wang B, Kang Y (2020) Novel coronavirus infection during the 2019–2020 epidemic: preparing intensive care units-the experience in Sichuan Province, China. Intensive Care Med 46(2):357–360
RKI (2020) Empfehlungen des Robert Koch-Institutes zu Hygienemaßnahmen im Rahmen der Behandlung von Patienten mit einer Infektion durch SARS-CoV‑2. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Hygiene.html. Zugegriffen: 9. März 2020
Arabi YM, Murthy S, Webb S (2020) COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med. https://doi.org/10.1007/s00134-020-05955-1
University of Liverpool (2020) Covid-19 drug interactions. http://www.covid19-druginteractions.org/. Zugegriffen: 9. März 2020
Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF (2020) Severe SARS-CoV‑2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. https://doi.org/10.1007/s00134-020-05967-x
Brunkhorst FM, Weigand MA, Pletz M, Gastmeier P, Lemmen SW, Meier-Hellmann A et al (2020) S3 guideline sepsis-prevention, diagnosis, treatment, and aftercare: Summary of the strong recommendations. Med Klin Intensivmed Notfmed 115(3):178–188. https://doi.org/10.1007/s00063-020-00671-6
WHO (2020) Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected Interim guidance. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf. Zugegriffen: 9. März 2020
Hui DS, Chow BK, Lo T, Tsang OTY, Ko FW, Ng SS et al (2019) Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. https://doi.org/10.1183/13993003.02339-2018
Cheung JC, Ho LT, Cheng JV, Cham EYK, Lam KN (2020) Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(20)30084-9
Fichtner F, Moerer O, Weber-Carstens S, Nothacker M, Kaisers U, Laudi S, Guideline group (2019) Clinical Guideline for Treating Acute Respiratory Insufficiency with Invasive Ventilation and Extracorporeal Membrane Oxygenation: Evidence-Based Recommendations for Choosing Modes and Setting Parameters of Mechanical Ventilation. Respiration 98(4):357–372. https://doi.org/10.1159/000502157
Acknowledgements
We thank Dr. Anne Mecklenburg for English translation and editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Kluge received research support by Ambu, E.T.View Ltd, Fisher & Paykel, Pfizer and Xenios. He also received lecture honorarium from ArjoHuntleigh, Astellas, Astra, Basilea, Bard, Baxter, Biotest, CSL Behring, Cytosorbents, Fresenius, Gilead, MSD, Orion, Pfizer, Philips, Sedana, Sorin, Xenios and Zoll. He received consultant honorarium from AMOMED, Astellas, Baxter, Bayer, Fresenius, Gilead, MSD, Pfizer and Xenios. U. Janssens has declared no conflict of interest. T. Welte received lecture honorarium and travel grants from Gilead. S. Weber-Carstens works in a research cooperation with Dräger. G. Marx received consultant honorarium and research support by Biotest, B.Braun und Adrenomed. C. Karagiannidis received consultant honoraria from Bayer und Xenios.
This article does not include human or animal studies conducted by the authors.
Rights and permissions
About this article
Cite this article
Kluge, S., Janssens, U., Welte, T. et al. German recommendations for critically ill patients with COVID‑19. Med Klin Intensivmed Notfmed 115 (Suppl 3), 111–114 (2020). https://doi.org/10.1007/s00063-020-00689-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00063-020-00689-w