Abstract
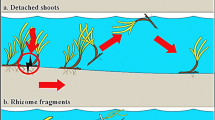
Facilitation (enhancement of propagule retention in this case) is increasingly recognized as an important driver of biodiversity, but it is still unknown if facilitation during dispersal and colonization is affected by self-organized spatial pattern formation. We investigated the ability of in-stream submerged macrophyte patches to trap the vegetative propagules of three species (Berula erecta, Groenlandia densa, Elodea nuttallii in two size classes: 13–22 and 40–48 cm long), and to potentially benefit the colonization of these three species. We tested the effects of propagule traits, hydrodynamic forcing, and spatial patch configuration on propagule trapping. Propagule buoyancy was negatively correlated with trapping chance, while propagule size did not influence trapping. Species-specific differences in buoyancy were maintained for weeks after fragmentation. Propagule retention was interactive and conditional upon the interplay between incoming flow velocities and vegetation spatial patterning. In the flume experiment at low flows, a patchy configuration (one patch filling 66% of the flume width) retained more surface-drifting propagules (B. erecta, G. densa), than near-homogeneous cover (two patches close together, filling the entire flume width). In contrast, retention of sinking E. nuttallii propagules increased in the two-patch configurations. In flume and field releases where patches did not completely fill the channel width, water flowed around the patches rather than over or through them. This resulted in low-flow velocity areas within patches where canopies were upright and propagules were retained, and higher velocity flows around patches. In contrast, when vegetation filled the channel width, water could not be diverted laterally around the patches and preferentially flowed over them, causing the canopies to bend and reduce their trapping capacity. In flume experiments at high flows, retention of all species decreased, regardless of vegetation configuration, as propagules passed over the reconfigured vegetation canopies. These findings on the interplay of water movement and patch reconfiguration suggest that environmental heterogeneity generated by the self-organizing behavior of aquatic plants might enhance colonization of sessile organisms, calling for landscape-scale processes like dispersal to be better investigated.
Similar content being viewed by others
Data Availabitiy
Data associated with this manuscript will be available from https://doi.org/10.4121/uuid:ce95f637-3487-4b24-81d4-2549c81e0ac0.
References
Aerts R, Maes W, November E, Behailu M, Poesen J, Deckers J, Hermy M, Muys B (2006) Surface runoff and seed trapping efficiency of shrubs in a regenerating semiarid woodland in northern Ethiopia. CATENA 65:61–70. https://doi.org/10.1016/j.catena.2005.09.004
Aguiar MR, Sala OE (1997) Seed distribution constrains the dynamics of the Patagonian steppe. Ecology 78:93–100
Barrat-Segretain M-H, Bornette G, Hering-Vilas-Bôas A (1998) Comparative abilities of vegetative regeneration among aquatic plants growing in disturbed habitats. Aquat Bot 60:201–211
Barrat-Segretain M-H, Henry CP, Bornette G (1999) Regeneration and colonization of aquatic plant fragments in relation to the disturbance frequency of their habitats. Archiv für Hydrobiologie 145:111–127
Barrat-Segretain M-H, Elger A, Sagnes P, Puijalon S (2002) Comparison of three life-history traits of invasive Elodea canadensis Michx. and Elodea nuttallii (Planch.) H. St John. Aquat Bot 74:299–313
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Bertness MD, Leonard GH (1997) The role of positive interactions in communities: lessons from intertidal habitats. Ecology 78:1976–1989
Bertness MD, Leonard GH, Levine JM, Schmidt PR, Ingraham AO (1999) Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology 80:2711–2726
Boedeltje G, Bakker JP, Bekker RM, Van Groenendael JM, Soesbergen M (2003) Plant dispersal in a lowland stream in relation to occurrence and three specific life-history traits of the species in the species pool. J Ecol 91:855–866
Boedeltje G, Bakker JP, Ten Brinke A, Van Groenendael JM, Soesbergen M (2004) Dispersal phenology of hydrochorous plants in relation to discharge, seed release time and buoyancy of seeds: the flood pulse concept supported. J Ecol 92:786–796
Bornette G, Puijalon S (2011) Response of aquatic plants to abiotic factors: a review. Aquat Sci 73:1–14
Borthagaray AI, Carranza A (2007) Mussels as ecosystem engineers: their contribution to species richness in a rocky littoral community. Acta Oecol 31:243–250
Bouma T, De Vries M, Low E, Peralta G, Tánczos I, van de Koppel J, Herman PMJ (2005) Trade-offs related to ecosystem engineering: a case study on stiffness of emerging macrophytes. Ecology 86:2187–2199
Bouma T, Van Duren L, Temmerman S, Claverie T, Blanco-Garcia A, Ysebaert T, Herman P (2007) Spatial flow and sedimentation patterns within patches of epibenthic structures: combining field, flume and modelling experiments. Cont Shelf Res 27:1020–1045
Bouma T, Friedrichs M, Klaassen P, van Kasenbeeck B, Brun F, Temmerman S, Van Katwijk M, Graf G, Herman P (2009) Effects of shoot stiffness, shoot size and current velocity on scouring sediment from around seedlings and propagules. Mar Ecol Prog Ser 388:293–297
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielbörger K, Travis JM, Anthelme F (2008) Facilitation in plant communities: the past, the present, and the future. J Ecol 96:18–34
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Callaway RM (1994) Facilitative and interfering effects of Arthrocnemum subterminale on winter annuals. Ecology 75:681–686
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349
Callaway RM (2007) Positive interactions and interdependence in plant communities. Springer, Dordrecht, The Netherlands
Carpenter SR, Lodge DM (1986) Effects of submersed macrophytes on ecosystem processes. Aquat Bot 26:341–370
Carthey AJ, Fryirs KA, Ralph TJ, Bu H, Leishman MR (2016) How seed traits predict floating times: a biophysical process model for hydrochorous seed transport behaviour in fluvial systems. Freshw Biol 61:19–31
Cellot B, Mouillot F, Henry CP (1998) Flood drift and propagule bank of aquatic macrophytes in a riverine wetland. J Veg Sci 9:631–640
Chadwell TB, Engelhardt KA (2008) Effects of pre-existing submersed vegetation and propagule pressure on the invasion success of Hydrilla verticillata. J Appl Ecol 45:515–523
Chang E, Veeneklaas R, Buitenwerf R, Bakker J, Bouma T (2008) To move or not to move: determinants of seed retention in a tidal marsh. Funct Ecol 22:720–727
Cook CD, Urmi-König K (1985) A revision of the genus Elodea (Hydrocharitaceae). Aquat Bot 21:111–156
Cornacchia L, Licci S, van de Koppel J, van der Wal D, Wharton G, Puijalon S, Bouma TJ (2016) Flow velocity and morphology of a submerged patch of the aquatic species Veronica anagallis-aquatica L. In: Rowiński PM, Marion A (eds) Hydrodynamic and mass transport at freshwater aquatic interfaces. Springer, Cham, pp 141–152
Cornacchia L, van de Koppel J, van der Wal D, Wharton G, Puijalon S, Bouma TJ (2018) Landscapes of facilitation: how self-organized patchiness of aquatic macrophytes promotes diversity in streams. Ecology 99:832–847. https://doi.org/10.1002/ecy.2177
Cotton J, Wharton G, Bass J, Heppell C, Wotton R (2006) The effects of seasonal changes to in-stream vegetation cover on patterns of flow and accumulation of sediment. Geomorphology 77:320–334
Dayton PK (1972) Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In: Proceedings of the colloquium on conservation problems in Antarctica, 1972. Allen Press Lawrence, KS, pp 81–96
Demars B, Gornall R (2003) Identification of British species of Callitriche by means of isozymes. Watsonia 24:389–400
Engström J, Nilsson C, Jansson R (2009) Effects of stream restoration on dispersal of plant propagules. J Appl Ecol 46:397–405
Eppinga MB, De Ruiter PC, Wassen MJ, Rietkerk M (2009) Nutrients and hydrology indicate the driving mechanisms of peatland surface patterning. Am Nat 173:803–818
Follett EM, Nepf HM (2012) Sediment patterns near a model patch of reedy emergent vegetation. Geomorphology 179:141–151
Fonseca MS, Zieman JC, Thayer GW, Fisher JS (1983) The role of current velocity in structuring eelgrass (Zostera marina L.) meadows Estuarine. Coast Shelf Sci 17:367–380
Gambi MC, Nowell AR, Jumars PA (1990) Flume observations on flow dynamics in Zostera marina (eelgrass) beds. Mar Ecol Prog Ser 61:159–169
Gillis L, Bouma T, Kiswara W, Ziegler A, Herman P (2014) Leaf transport in mimic mangrove forests and seagrass beds. Mar Ecol Prog Ser 498:95–102
Goodson J, Gurnell A, Angold P, Morrissey I (2001) Riparian seed banks: structure, process and implications for riparian management. Prog Phys Geogr 25:301–325
Goodson J, Gurnell A, Angold P, Morrissey I (2003) Evidence for hydrochory and the deposition of viable seeds within winter flow-deposited sediments: the River Dove, Derbyshire. UK River Res Appl 19:317–334
Granata T, Serra T, Colomer J, Casamitjana X, Duarte C, Gacia E (2001) Flow and particle distributions in a nearshore seagrass meadow before and after a storm. Mar Ecol Progress Ser 218:95–106
Gurnell AM (2007) Analogies between mineral sediment and vegetative particle dynamics fluvial systems. Geomorphology 89:9–22. https://doi.org/10.1016/j.geomorph.2006.07.012
Gurnell AM, O’Hare MT, O’Hare JM, Scarlett P, Liffen TM (2013) The geomorphological context and impact of the linear emergent macrophyte, Sparganium erectum L.: a statistical analysis of observations from. Br Rivers Earth Surf Process Landf 38:1869–1880
Haslam SM (1978) River plants: the macrophyte vegetation of watercourses. Cambridge Univer Press, Cambridge
Hiemstra CA, Liston GE, Reiners WA (2002) Snow redistribution by wind and interactions with vegetation at upper treeline in the Medicine Bow Mountains, Wyoming, USA. Arct Antarctic Alp Res 34:262–273
Johansson ME, Nilsson C (1993) Hydrochory, population dynamics and distribution of the clonal aquatic plant Ranunculus lingua. J Ecol 81:81–91
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. In: Samson FB, Knopf FL (eds) Ecosystem management. Springer, New York, NY, pp 130–147
Kondziolka JM, Nepf HM (2014) Vegetation wakes and wake interaction shaping aquatic landscape evolution Limnology and Oceanography. Fluids Environ 4:106–119
Larsen LG, Harvey JW (2010) How vegetation and sediment transport feedbacks drive landscape change in the Everglades and wetlands worldwide. Am Nat 176:E66–E79
Larsen LG, Harvey JW, Crimaldi JP (2007) A delicate balance: ecohydrological feedbacks governing landscape morphology in a lotic peatland. Ecol Monogr 77:591–614
Licci S, Delolme C, Marmonier P, Philippe M, Cornacchia L, Gardette V, Bouma T, Puijalon S (2016) Effect of aquatic plant patches on flow and sediment characteristics: the case of Callitriche platycarpa and Elodea nuttallii. In: Rowiński M, Marion P A (eds) Hydrodynamic and mass transport at freshwater aquatic interfaces: 34th International School of Hydraulics. Springer International Publishing, Cham, pp 129–140. https://doi.org/10.1007/978-3-319-27750-9_11
Malmqvist B (2002) Aquatic invertebrates in riverine landscapes. Freshw Biol 47:679–694
McIntire EJ, Fajardo A (2014) Facilitation as a ubiquitous driver of biodiversity. New Phytol 201:403–416
McKee KL, Rooth JE, Feller IC (2007) Mangrove recruitment after forest disturbance is facilitated by herbaceous species in the Caribbean. Ecol Appl 17:1678–1693. https://doi.org/10.1890/06-1614.1
Merritt DM, Wohl EE (2002) Processes governing hydrochory along rivers: hydraulics, hydrology, and dispersal phenology. Ecol Appl 12:1071–1087
Minckley W (1963) The ecology of a spring stream: Doe Run, Meade County, Kentucky. Wildl Monogr 11:3–124
Niering W, Whittaker R, Lowe C (1963) The saguaro: a population in relation to environment. Science 142:15–23
Nilsson C, Brown RL, Jansson R, Merritt DM (2010) The role of hydrochory in structuring riparian and wetland vegetation. Biol Rev 85:837–858
O’Hare JM, O’Hare MT, Gurnell AM, Scarlett PM, Liffen T, McDonald C (2012) Influence of an ecosystem engineer, the emergent macrophyte Sparganium erectum, on seed trapping in lowland rivers and consequences for landform colonisation. Freshw Biol 57:104–115
Padilla FM, Pugnaire FI (2006) The role of nurse plants in the restoration of degraded environments. Front Ecol Environ 4:196–202
Peterson JM, Bell SS (2012) Tidal events and salt-marsh structure influence black mangrove (Avicennia germinans) recruitment across and ecotone. Ecology 93:1648–1658
Pueyo Y, Kefi S, Alados C, Rietkerk M (2008) Dispersal strategies and spatial organization of vegetation in arid ecosystems. Oikos 117:1522–1532
R Core Team (2015) R: a language and environment for statistical computing (Version 3.1. 2): R Foundation for Statistical Computing
Rabinowitz D (1978) Dispersal properties of mangrove propagules. Biotropica 10:47–57
Rietkerk M, van de Koppel J (2008) Regular pattern formation in real ecosystems. Trends Ecol Evol 23:169–175
Riis T (2008) Dispersal and colonisation of plants in lowland streams: success rates bottlenecks. Hydrobiologia 596:341–351
Riis T, Biggs BJF (2003) Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnol Oceanogr 48:1488–1497
Riis T, Sand-Jensen K (2006) Dispersal of plant fragments in small streams. Freshw Biol 51:274–286
Sand-Jensen K (1998) Influence of submerged macrophytes on sediment composition and near-bed flow in lowland streams. Freshw Biol 39:663–679
Sand-Jensen K, Mebus JR (1996) Fine-scale patterns of water velocity within macrophyte patches in streams. Oikos 76:169–180
Sand-Jensen K, Pedersen ML (2008) Streamlining of plant patches in streams. Freshw Biol 53:714–726
Sand-Jensen K, Andersen K, Andersen T (1999) Dynamic properties of recruitment, expansion and mortality of macrophyte patches in streams. Int Rev Hydrobiol 84:497–508
Sarneel J (2013) The dispersal capacity of vegetative propagules of riparian fen species. Hydrobiologia 710:219–225
Säumel I, Kowarik I (2013) Propagule morphology and river characteristics shape secondary water dispersal in tree species. Plant Ecol 214:1257–1272
Schoelynck J, De Groote T, Bal K, Vandenbruwaene W, Meire P, Temmerman S (2012) Self-organised patchiness and scale-dependent bio-geomorphic feedbacks in aquatic river vegetation. Ecography 35:760–768
Schoelynck J, Meire D, Bal K, Buis K, Troch P, Bouma T, Meire P, Temmerman S (2013) Submerged macrophytes avoiding a negative feedback in reaction to hydrodynamic stress. Limnol Ecol Manag Inland Waters 43:371–380
Sculthorpe CD (1967) Biology of aquatic vascular plants. St. Martin’s, New York
Soomers H, Karssenberg D, Soons MB, Verweij PA, Verhoeven JT, Wassen MJ (2013) Wind and water dispersal of wetland plants across fragmented landscapes. Ecosystems 16:434–451
Soons MB, Groot GA, Cuesta Ramirez MT, Fraaije RG, Verhoeven JT, Jager M (2017) Directed dispersal by an abiotic vector: Wetland plants disperse their seeds selectively to suitable sites along the hydrological gradient via water. Funct Ecol 31:499–508
Temmerman S, Bouma T, Van de Koppel J, Van der Wal D, De Vries M, Herman P (2007) Vegetation causes channel erosion in a tidal landscape. Geology 35:631–634
Thomaz SM, Mormul RP, Michelan TS (2015) Propagule pressure, invasibility of freshwater ecosystems by macrophytes and their ecological impacts: a review of tropical freshwater ecosystems. Hydrobiologia 746:39–59
Turner T (1983) Facilitation as a successional mechanism in a rocky intertidal community. Am Nat 121:729–738
van de Koppel J, Altieri AH, Silliman BR, Bruno JF, Bertness MD (2006) Scale-dependent interactions and community structure on cobble beaches. Ecol Lett 9:45–50
Van Der Heide T, Bouma TJ, Van Nes EH, Van De Koppel J, Scheffer M, Roelofs JG, Van Katwijk MM, Smolders AJ (2010) Spatial self-organized patterning in seagrasses along a depth gradient of an intertidal ecosystem. Ecology 91:362–369
Van der Stocken T, De Ryck D, Vanschoenwinkel BB, Bouma T, Dahdouh-Guebas F, Koedam N (2015) Impact of landscape structure on propagule dispersal in mangrove forests. Mar Ecol Prog Ser 524:95–106
Vandenbruwaene W, Temmerman S, Bouma T, Klaassen P, De Vries M, Callaghan D, Van Steeg P, Dekker F, Van Duren L, Martini E (2011) Flow interaction with dynamic vegetation patches: implications for biogeomorphic evolution of a tidal landscape. J Geophys Res Earth Surf 116:F01008
Weerman EJ, Van de Koppel J, Eppinga MB, Montserrat F, Liu QX, Herman PM (2010) Spatial self-organization on intertidal mudflats through biophysical stress divergence. Am Nat 176:E15–E32
Wharton G, Cotton JA, Wotton RS, Bass JA, Heppell CM, Trimmer M, Sanders IA, Warren LL (2006) Macrophytes and suspension-feeding invertebrates modify flows and fine sediments in the Frome and Piddle catchments, Dorset (UK). J Hydrol 330:171–184
Acknowledgements
This work was supported by the Research Executive Agency, through the Seventh Framework Programme of the European Union, Support for Training and Career Development of Researchers (Marie Curie - FP7-PEOPLE-2012-ITN), which funded the Initial Training Network (ITN) HYTECH ‘Hydrodynamic Transport in Ecologically Critical Heterogeneous Interfaces’, N.316546. We thank the CNR (Compagnie Nationale du Rhône) for providing access to field sites. We thank the Associate Editor and two anonymous referees for their helpful comments that have improved the quality of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cornacchia, L., van der Wal, D., van de Koppel, J. et al. Flow-divergence feedbacks control propagule retention by in-stream vegetation: the importance of spatial patterns for facilitation. Aquat Sci 81, 17 (2019). https://doi.org/10.1007/s00027-018-0612-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-018-0612-1