Abstract
Objective and design
To understand the expression of dsRNA-dependent protein kinase R (PKR) in impaired diabetic wounds, hyperglycemia was induced in C57/BL6 mice with streptozotocin. Murine macrophage cell line, Raw 264.7, stimulated with high glucose and LPS was used to mimic diabetic wound environment in in-vitro.
Materials
Macrophages stimulated with HG + LPS, in presence and absence of PKR inhibitor (C16) and wound tissue samples from topically treated mice with C16, were analyzed for the expression of PKR, NALP3, active caspase-1, mature IL-1β and phosphorylation of PKR and eIF2α. Wounds tissues were also analyzed for inflammatory cell infiltration by immunohistochemistry, angiogenesis by CD31 staining, collagen expression by western blotting, expression of CD206+ macrophages by flow cytometry and wound strength by texture analyzer.
Results
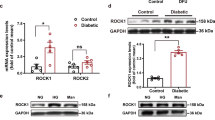
PKR and NALP3 were found to be upregulated in macrophages stimulated with HG + LPS as well as in impaired diabetic wounds. PKR inhibition using C16 ameliorated expression of NALP3, caspase-1, IL-1β and phosphorylation of PKR and eIF2α, in macrophages and also in diabetic wounds. Treatment with C16 promoted the wound healing in diabetic mice by increasing collagen synthesis, reducing infiltration of F4/80+ macrophages and MPO+ neutrophil cells, increased angiogenesis, and increased number of M2 macrophages.
Conclusion
PKR inhibition using C16 accelerates the wound healing process in diabetic mice by decreasing NALP3-mediated IL-1β maturation.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PKR:
-
Double-stranded RNA-dependent protein kinase
- NALP3:
-
NACHT, LRR and PYD domains containing protein 3
- MPO:
-
Myeloperoxidase
- CD31:
-
Cluster of differentiation 31 also named as PECAM-1
- PECAM-1:
-
Platelet endothelial cell adhesion molecule
References
Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63:1103–14.
Kant V, Gopal A, Pathak NN, Kumar P, Tandan SK, Kumar D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int Immunopharmacol. 2014;20:322–30. https://doi.org/10.1016/j.intimp.2014.03.009.
Okonkwo UA, Dipietro LA. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18:1–15.
Mirza RE, Fang MM, Ennis WJ, Kohl TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62:2579–87.
Weinheimer-Haus EM, Mirza RE, Koh TJ. Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing. PLoS ONE. 2015;10:1–13.
Karnam K, Sedmaki K, Sharma P, Routholla G, Goli S, Ghosh B, et al. HDAC6 inhibitor accelerates wound healing by inhibiting tubulin mediated IL-1β secretion in diabetic mice. Biochim Biophys Acta Mol Basis Dis. 2020. https://doi.org/10.1016/j.bbadis.2020.165903.
Bitto A, Altavilla D, Pizzino G, Irrera N, Pallio G, Colonna MR, et al. Inhibition of inflammasome activation improves the impaired pattern of healing in genetically diabetic mice. Br J Pharmacol. 2014;171:2300–7.
Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1ß, a protein lacking a signal sequence. EMBO J. 1990;9:1503–10.
Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32.
Van De VFL, Netea MG, Dinarello CA, Joosten LAB. Inflammasome activation and IL-1 b and IL-18 processing during infection. Trends Immunol. 2011. https://doi.org/10.1016/j.it.2011.01.003.
Diamond C, Zeitler M, Gomez AI, Bagnall J, Spiller D, White M, et al. Inflammasome-dependent IL-1 β release depends upon membrane permeabilisation. Cell Death Differ. 2016;23:1219–31.
Burm SM, Zuiderwijk-Sick EA, ’t Jong AEJ, Van Der Putten C, Veth J, Kondova I, et al. Inflammasome-induced IL-1β secretion in microglia is characterized by delayed kinetics and is only partially dependent on inflammatory caspases. J Neurosci. 2015;35:678–87.
McGettrick AF, O’Neill LA. NLRP3 and IL-1β in macrophages as critical regulators of metabolic diseases. Diabetes Obes Metab. 2013;15(Suppl 3):19–25. https://doi.org/10.1111/dom.12169 (PMID: 24003917).
Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204.
Zhang X, Dai J, Li L, Chen H, Chai Y. NLRP3 inflammasome expression and signaling in human diabetic wounds and in high glucose induced macrophages. J Diabetes Res. 2017. https://doi.org/10.1155/2017/5281358.
Lupfer C, Malik A, Kanneganti TD. Inflammasome control of viral infection. Curr Opin Virol. 2015;12:38–46.
Meurs E, Chong K, Galabru J, Thomas NSB, Kerr IM, Williams BRG, et al. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–90.
Koromilas AE, Roy S, Barber GN, Katze MG, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992;257:1685–9.
Balachandran S, Barber GN. PKR in innate immunity, cancer, and viral oncolysis. Methods Mol Biol. 2007;383:277–301.
García MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, et al. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006;70:1032–60.
Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–48.
Nakamura T, Arduini A, Baccaro B, Furuhashi M, Hotamisligil GS. Small-molecule inhibitors of PKR improve glucose homeostasis in obese diabetic mice. Diabetes. 2014;63:526–34.
Carvalho-Filho MA, Carvalho BM, Oliveira AG, Guadagnini D, Ueno M, Dias MM, et al. Double-stranded RNA-activated protein kinase is a key modulator of insulin sensitivity in physiological conditions and in obesity in mice. Endocrinology. 2012;153:5261–74.
Mangali S, Bhat A, Jadhav K, Kalra J, Sriram D, Vamsi Krishna Venuganti V, et al. Upregulation of PKR pathway mediates glucolipotoxicity induced diabetic cardiomyopathy in vivo in wistar rats and in vitro in cultured cardiomyocytes. Biochem Pharmacol. 2020;177:113948. https://doi.org/10.1016/j.bcp.2020.113948.
Udumula MP, Mangali S, Kalra J, Dasari D, Goyal S, Krishna V, et al. High fructose and streptozotocin induced diabetic impairments are mitigated by Indirubin-3-hydrazone via downregulation of PKR pathway in Wistar rats. Sci Rep. 2021;11:1–11. https://doi.org/10.1038/s41598-021-92345-2.
Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundbäck P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488(7413):670–4.
Mangali S, Bhat A, Udumula MP, Dhar I, Sriram D, Dhar A. Inhibition of protein kinase R protects against palmitic acid–induced inflammation, oxidative stress, and apoptosis through the JNK/NF-kB/NLRP3 pathway in cultured H9C2 cardiomyocytes. J Cell Biochem. 2019;120:3651–63.
Li Y, Li Y, Li L, Yin M, Wang J, Li X. PKR deficiency alleviates pulmonary hypertension via inducing inflammasome adaptor ASC inactivation. Pulm Circ. 2021;11:1–13.
Jiang Y, Steinle JJ. Epac1 inhibits PKR to reduce NLRP3 inflammasome proteins in retinal endothelial cells. J Inflamm Res. 2019;12:153–9.
Yim HCH, Wang D, Yu L, White CL, Faber PW, Williams BRG, et al. The kinase activity of PKR represses inflammasome activity. Cell Res. 2016;26:367–79. https://doi.org/10.1038/cr.2016.11.
Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70:5.47.1-5.47.20.
Corso CO, Gundersen Y, Dörger M, Lilleaasen P, Aasen AO, Messmer K. Effects of the nitric oxide synthase inhibitors N(G)-nitro-L-arginine methyl ester and aminoethyl-isothiourea on the liver microcirculation in rat endotoxemia. J Hepatol. 1998;28:61–9.
Kulkarni NN, Adase CA, Zhang L, Borkowski AW, Li F, Sanford JA, et al. IL-1 receptor e knockout mice develop epidermal cysts and show an altered innate immune response after exposure to UVB radiation. J Invest Dermatol. 2017;137:2417–26.
Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23:594–608.
Zhu Z, Chen AF. GW26-e5321 Inhibition of PKR impairs angiogenesis through a VEGF pathway. J Am Coll Cardiol. 2015;66:C17. https://doi.org/10.1016/j.jacc.2015.06.1094.
Acknowledgements
The authors would like to thank Central Analytical (CAL) facility at BITS-PILANI, Hyderabad campus for providing the access to use laser confocal microscopy (Leica DMi8), BD FACS Aria™ III and HPLC.
Funding
This study was funded by Department of Science and technology, India (DST-SERB) [YSS/2015/001969] to O.P.K. O.P.K also received funds from BITS-PILANI, Hyderabad campus (Additional research initiation grant).
Author information
Authors and Affiliations
Contributions
OPK conceived the idea and OPK, VVVK and KK designed the experiments. KK, SK and PS performed the experiments and analyzed the data. KK and OPK completed the manuscript writing. All the authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The animal studies performed in this report were approved by institute animal ethical committee (BITS-HYD/IAEC/2016/02) and (BITS-HYD/IAEC/2018/12).
Additional information
Responsible Editor: Mauro Teixeira.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karnam, K., Sedmaki, K., Sharma, P. et al. Selective inhibition of PKR by C16 accelerates diabetic wound healing by inhibiting NALP3 expression in mice. Inflamm. Res. 72, 221–236 (2023). https://doi.org/10.1007/s00011-022-01667-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01667-y