Abstract
Background
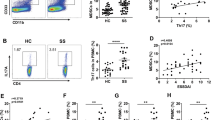
Recent studies have revealed a role of the ligand for glucocorticoid-induced TNFR family-related protein (GITRL) in mediating functional dysregulations of myeloid-derived suppressor cells (MDSCs) in the pathogenesis of primary Sjögren syndrome (pSS), but the underlying molecular mechanism is largely unclear. In this study, we aimed to elucidate GITRL-mediated signaling pathways in MDSCs during the development of experimental SS (ESS).
Methods
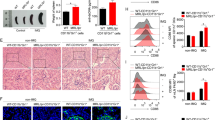
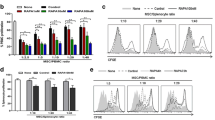
MDSCs were stimulated with recombinant GITRL, the activation of PTEN, AKT and STAT3 in MDSCs was analyzed by Western blot. MDSCs with different treatment were adoptively transferred to ESS mice. ELISA was used to detect the level of autoantibodies. Proportions of Th1 and Th17 cells were examined by flow cytometry. Histological evaluation of glandular destruction was analyzed by hematoxylin and eosin (HE) staining. The interaction of GITR, TRAF3 and PP2A was detected by CoIP.
Results
Upon the engagement of GITR on MDSCs, PTEN was activated and led to the inhibition of downstream AKT/STAT3 signaling pathway, therefore, resulting in the impaired immunosuppressive function of MDSCs. In ESS mice, blocking the activity of PTEN could efficiently restore the immunomodulatory effect of MDSCs and alleviate the progression of ESS. Furthermore, TRAF3 was found to bind to GITR, and then recruited PP2A to dephosphorylate PTEN, thus enhancing the activity of PTEN.
Conclusion
This study elucidated the molecular mechanism underlying the effect of GITRL in regulating the function of MDSCs, which may provide a new therapeutic target for the treatment of pSS.





Similar content being viewed by others
References
Brito-Zeron P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, et al. Sjogren syndrome. Nat Rev Dis Primers. 2016;2:16047. https://doi.org/10.1038/nrdp.2016.47.
Fox RI. Sjögren’s syndrome. The Lancet. 2005;366:321–31. https://doi.org/10.1016/s0140-6736(05)66990-5.
Mariette X, Criswell LA. Primary Sjogren’s syndrome. N Engl J Med. 2018;378:931–9. https://doi.org/10.1056/NEJMcp1702514.
Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76:9–16. https://doi.org/10.1136/annrheumdis-2016-210571.
Mona M, Mondello S, Hyon JY, Saleh W, Han K, Lee HJ, et al. Clinical usefulness of anti-muscarinic type 3 receptor autoantibodies in patients with primary Sjogren’s syndrome. Clin Exp Rheumatol. 2021;39:795–803. https://doi.org/10.55563/clinexprheumatol/gy6udz.
Tsuboi H, Matsumoto I, Wakamatsu E, Nakamura Y, Iizuka M, Hayashi T, et al. New epitopes and function of anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjogren’s syndrome. Clin Exp Immunol. 2010;162:53–61. https://doi.org/10.1111/j.1365-2249.2010.04188.x.
Dawson LJ, Stanbury J, Venn N, Hasdimir B, Rogers SN, Smith PM. Antimuscarinic antibodies in primary Sjogren’s syndrome reversibly inhibit the mechanism of fluid secretion by human submandibular salivary acinar cells. Arthritis Rheum. 2006;54:1165–73. https://doi.org/10.1002/art.21764.
Xiao F, Han M, Rui K, Ai X, Tian J, Zhang W, et al. New insights into follicular helper T cell response and regulation in autoimmune pathogenesis. Cell Mol Immunol. 2021;18:1610–2. https://doi.org/10.1038/s41423-021-00688-7.
Tian J, Hong Y, Zhu Q, Zhou H, Zhang Y, Shen Z, et al. Mesenchymal stem cell enhances the function of MDSCs in experimental Sjogren syndrome. Front Immunol. 2020;11:604607. https://doi.org/10.3389/fimmu.2020.604607.
Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjogren’s syndrome. Nat Rev Rheumatol. 2013;9:544–56. https://doi.org/10.1038/nrrheum.2013.110.
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021. https://doi.org/10.1038/s41577-020-00490-y.
Guo C, Hu F, Yi H, Feng Z, Li C, Shi L, et al. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Ann Rheum Dis. 2016;75:278–85. https://doi.org/10.1136/annrheumdis-2014-205508.
Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189:4295–304. https://doi.org/10.4049/jimmunol.1200086.
Wu H, Zhen Y, Ma Z, Li H, Yu J, Xu ZG, et al. Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci Transl Med. 2016;8:331ra40. https://doi.org/10.1126/scitranslmed.aae0482.
Tian J, Rui K, Hong Y, Wang X, Xiao F, Lin X, et al. Increased GITRL impairs the function of myeloid-derived suppressor cells and exacerbates primary Sjögren syndrome. J Immunol. 2019;202:1693–703. https://doi.org/10.4049/jimmunol.1801051.
Trikha P, Carson WE 3rd. Signaling pathways involved in MDSC regulation. Biochim Biophys Acta. 2014;1846:55–65. https://doi.org/10.1016/j.bbcan.2014.04.003.
Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–9. https://doi.org/10.1172/JCI60083.
Mao FY, Zhao YL, Lv YP, Teng YS, Kong H, Liu YG, et al. CD45(+)CD33(low)CD11b(dim) myeloid-derived suppressor cells suppress CD8(+) T cell activity via the IL-6/IL-8-arginase I axis in human gastric cancer. Cell Death Dis. 2018;9:763. https://doi.org/10.1038/s41419-018-0803-7.
Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Colafrancesco S, et al. Phosphatidylinositol 3-kinase delta pathway: a novel therapeutic target for Sjogren’s syndrome. Ann Rheum Dis. 2019;78:249–60. https://doi.org/10.1136/annrheumdis-2017-212619.
Hong X, Wang X, Rang X, Yin X, Zhang X, Wang R, et al. The shared mechanism and candidate drugs of multiple sclerosis and Sjogren’s syndrome analyzed by bioinformatics based on GWAS and transcriptome data. Front Immunol. 2022;13:857014. https://doi.org/10.3389/fimmu.2022.857014.
Rui K, Hong Y, Zhu Q, Shi X, Xiao F, Fu H, et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate murine Sjogren’s syndrome by modulating the function of myeloid-derived suppressor cells. Cell Mol Immunol. 2021;18:440–51. https://doi.org/10.1038/s41423-020-00587-3.
Tian J, Zhang B, Rui K, Wang S. The role of GITR/GITRL interaction in autoimmune diseases. Front Immunol. 2020;11:588682. https://doi.org/10.3389/fimmu.2020.588682.
Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172:4686–90. https://doi.org/10.4049/jimmunol.172.8.4686.
Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–22. https://doi.org/10.1002/eji.200324804.
Petrillo MG, Ronchetti S, Ricci E, Alunno A, Gerli R, Nocentini G, et al. GITR+ regulatory T cells in the treatment of autoimmune diseases. Autoimmun Rev. 2015;14:117–26. https://doi.org/10.1016/j.autrev.2014.10.011.
Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. https://doi.org/10.1038/ni759.
Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. https://doi.org/10.1038/nri910.
Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. https://doi.org/10.1146/annurev.immunol.20.091301.131133.
Li H, Hostager BS, Arkee T, Bishop GA. Multiple mechanisms for TRAF3-mediated regulation of the T cell costimulatory receptor GITR. J Biol Chem. 2021;297:101097. https://doi.org/10.1016/j.jbc.2021.101097.
Esparza EM, Arch RH. Glucocorticoid-induced TNF receptor, a costimulatory receptor on naive and activated T cells, uses TNF receptor-associated factor 2 in a novel fashion as an inhibitor of NF-kappa B activation. J Immunol. 2005;174:7875–82. https://doi.org/10.4049/jimmunol.174.12.7875.
Esparza EM, Arch RH. TRAF4 functions as an intermediate of GITR-induced NF-kappaB activation. Cell Mol Life Sci. 2004;61:3087–92. https://doi.org/10.1007/s00018-004-4417-0.
Wang S, Shi Y, Yang M, Ma J, Tian J, Chen J, et al. Glucocorticoid-induced tumor necrosis factor receptor family-related protein exacerbates collagen-induced arthritis by enhancing the expansion of Th17 cells. Am J Pathol. 2012;180:1059–67. https://doi.org/10.1016/j.ajpath.2011.11.018.
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. https://doi.org/10.1126/science.275.5308.1943.
Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol 2018; 19:547–562. https://doi.org/10.1038/s41580-018-0015-0.
Nocentini G, Riccardi C. GITR: a modulator of immune response and inflammation. Adv Exp Med Biol. 2009;647:156–73. https://doi.org/10.1007/978-0-387-89520-8_11.
Wallis AM, Bishop GA. TRAF3 regulation of inhibitory signaling pathways in B and T lymphocytes by kinase and phosphatase localization. J Leukoc Biol. 2018. https://doi.org/10.1002/JLB.2MIR0817-339RR.
Nakahata S, Ichikawa T, Maneesaay P, Saito Y, Nagai K, Tamura T, et al. Loss of NDRG2 expression activates PI3K-AKT signalling via PTEN phosphorylation in ATLL and other cancers. Nat Commun. 2014;5:3393. https://doi.org/10.1038/ncomms4393.
Fujii W, Ashihara E, Hirai H, Nagahara H, Kajitani N, Fujioka K, et al. Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J Immunol. 2013;191:1073–81. https://doi.org/10.4049/jimmunol.1203535.
Ioannou M, Alissafi T, Lazaridis I, Deraos G, Matsoukas J, Gravanis A, et al. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2012;188:1136–46. https://doi.org/10.4049/jimmunol.1101816.
Park MJ, Lee SH, Kim EK, Lee EJ, Park SH, Kwok SK, et al. Myeloid-derived suppressor cells induce the expansion of regulatory B cells and ameliorate autoimmunity in the Sanroque mouse model of systemic lupus erythematosus. Arthritis Rheumatol. 2016;68:2717–27. https://doi.org/10.1002/art.39767.
Zhang H, Huang Y, Wang S, Fu R, Guo C, Wang H, et al. Myeloid-derived suppressor cells contribute to bone erosion in collagen-induced arthritis by differentiating to osteoclasts. J Autoimmun. 2015;65:82–9. https://doi.org/10.1016/j.jaut.2015.08.010.
Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–37.
Gan X, Feng X, Gu L, Tan W, Sun X, Lv C, et al. Correlation of increased blood levels of GITR and GITRL with disease severity in patients with primary Sjogren’s syndrome. Clin Dev Immunol. 2013;2013:340751. https://doi.org/10.1155/2013/340751.
Jiang M, Chen J, Zhang W, Zhang R, Ye Y, Liu P, et al. Interleukin-6 trans-signaling pathway promotes immunosuppressive myeloid-derived suppressor cells via suppression of suppressor of cytokine signaling 3 in breast cancer. Front Immunol. 2017;8:1840. https://doi.org/10.3389/fimmu.2017.01840.
Hix LM, Karavitis J, Khan MW, Shi YH, Khazaie K, Zhang M. Tumor STAT1 transcription factor activity enhances breast tumor growth and immune suppression mediated by myeloid-derived suppressor cells. J Biol Chem. 2013;288:11676–88. https://doi.org/10.1074/jbc.M112.441402.
Colafrancesco S, Ciccacci C, Priori R, Latini A, Picarelli G, Arienzo F, et al. STAT4, TRAF3IP2, IL10, and HCP5 polymorphisms in Sjogren’s syndrome: association with disease susceptibility and clinical aspects. J Immunol Res. 2019;2019:7682827. https://doi.org/10.1155/2019/7682827.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81971542, 82171771, 82271854, 82004171, 82071817), Young Elite Scientist Sponsorship Program by CACM (CACM-2020-QNRC2-05), Hong Kong Research Grants Council General Research Fund (17113319, 17103821, 17111222) and Theme-Based Research Scheme (T12-703/19R), Shenzhen Science and Technology Program (YCYJ20210324114602008).
Author information
Authors and Affiliations
Contributions
JT and BZ performed the experiments, analyzed the data, and wrote the paper; QY, XS and CL performed the experiments; NP, BZ, MH, FX, WX and MC analyzed the data; KR, SW and LL designed the study and revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no financial conflicts of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, J., Zhang, B., Yuan, Q. et al. GITRL impairs the immunosuppressive function of MDSCs via PTEN-mediated signaling pathway in experimental Sjögren syndrome. Inflamm. Res. 71, 1577–1588 (2022). https://doi.org/10.1007/s00011-022-01660-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01660-5