Abstract
Introduction
This study investigated the mechanism of action of a synthetic tetrahydroisoquinoline alkaloid, MHTP, in an experimental model of acute lung injury (ALI) in two distinct moments: 72 h and 10 days.
Methodology
To realize this study, 2.5 mg/kg of lipopolysaccharide (LPS) was intranasally administered in BALB/c mice, and nasal instillation of MHTP (1.25; 2.5; 5.0; 10 or 20 mg/kg) was administrated at 1, 24, and 48 h after LPS challenge. The data were statistically analyzed and p < 0.05 was considered statistically significant.
Results
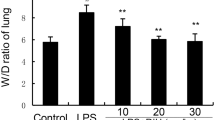
MHTP treatment (2.5, 5.0, 10 or 20 mg/kg) significantly decreased neutrophil migration into the bronchoalveolar lavage fluid (BALF), tissue inflammatory cell infiltration, edema, and hemorrhage as well as collagen fiber deposition on the perialveolar regions at both moments. TNF-α and IL-6 levels were significantly diminished in the MHTP-treated animals at 72 h and maintained them, at a basal level, at 10-day observation. These effects of MHTP are due to downregulating p38MAPkinese/p65NFκB signaling pathway-TLR4 dependent. Also, the MHTP treatment promoted a survival rate at 100% and improved their body weights during the 10-day observation. Unlike, the LPS group (non-treated LPS challenged animals) presented less than 50% of surviving rate at 72 h and the animals that survived did not improve their physiological state at 10-day observation.
Conclusions
These data showed for the first time the beneficial and effective activity of a nasal treatment with a synthetic tetrahydroisoquinoline alkaloid on an experimental model of ALI and pointed out the molecular mechanism related to it.





Similar content being viewed by others
References
Bhadra K, Kumar GS. Therapeutic potential of nucleic acid-binding tetrahydroisoquinoline alkaloids: binding aspects and implications for drug design. Med Res Rev. 2011;31:821–62.
Kuo Y-C, Lin W-C, Chiang I-T, Chang Y-F, Chen C-W, Su S-H, et al. Sorafenib sensitizes human colorectal carcinoma to radiation via suppression of NF-κB expression in vitro and in vivo. Biomed Pharmacother [Internet]. 2012 [cited 2019 Mar 19];66:12–20. http://www.ncbi.nlm.nih.gov/pubmed/22265104.
Wan J, Liu T, Mei L, Li J, Gong K, Yu C, et al. Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling. Br J Cancer [Internet]. Nature Publishing Group; 2013 [cited 2019 Mar 19];109:342–50. http://www.ncbi.nlm.nih.gov/pubmed/23807172.
Bae D-S, Kim Y-H, Pan C-H, Nho C-W, Samdan J, Yansan J, et al. Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages. BMB Rep [Internet]. 2012 [cited 2019 Mar 19];45:108–13. http://www.ncbi.nlm.nih.gov/pubmed/22360889.
Hu Y, Mao A, Yu Z, He K. Anti-endotoxin and anti-inflammatory effects of Chinese herbal medicinal alkaloid ingredients in vivo. Microb Pathog [Internet]. 2016 [cited 2019 Mar 19];99:51–5. http://www.ncbi.nlm.nih.gov/pubmed/27498361.
Frick S, Kramell R, Schmidt J, Fist AJ, Kutchan TM. Comparative qualitative and quantitative determination of alkaloids in narcotic and condiment Papaver somniferum cultivars. J Nat Prod. 2005;68:666–73.
Dang TTT, Onoyovwi A, Farrow SC, Facchini PJ. Biochemical genomics for gene discovery in benzyltetrahydroisoquinoline alkaloid biosynthesis in opium poppy and related species. Methods Enzymol [Internet]. Academic Press; 2012 [cited 2018 May 1];515:231–66. https://www.sciencedirect.com/science/article/pii/B9780123942906000112.
Goldstein LH, Weber-Schöndorfer C, Berkovitch M. Antiasthmatic and cough medication. Drugs Dur Pregnancy Lact [Internet]. Elsevier; 2015 [cited 2018 May 1]. p. 65–74. http://linkinghub.elsevier.com/retrieve/pii/B9780124080782000044.
Minor DL, Wyrick SD, Charifson PS, Watts VJ, Nichols DE, Mailman RB. Synthesis and molecular modeling of 1-phenyl-1,2,3,4-tetrahydrotetrahydroisoquinolines and related 5,6,8,9-tetrahydro-13bH-dibenzo[a,h]quinolizines as D1 dopamine antagonists. J Med Chem [Internet]. 1994 [cited 2019 Mar 19];37:4317–28. http://www.ncbi.nlm.nih.gov/pubmed/7996543.
Munchhof MJ, Meyers AI. A novel route to chiral, nonracemic 1-alkyl- and 1-aryl-substituted tetrahydrotetrahydroisoquinolines. Synthesis of (−)-salsolidine and (+)-cryptostyline II. J Org Chem. 1995;60:7086–7.
Kang YJ, Lee BK, Lee YS, Seo HG, Park MK, Kim HJ, et al. Suppression of tumor necrosis factor-α and inducible nitric oxide synthase gene expression by THI 52, a new synthetic naphthyl-benzyltetrahydroisoquinoline alkaloid. Biochem Pharmacol. 2003;65:457–64.
Tsoyi K, Kim HJ, Shin JS, Kim DH, Cho HJ, Lee SS, et al. HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydrotetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cell Signal. 2008;20:1839–47.
Pacheco de Oliveira MT, de Oliveira Ramalho TR, Paiva Ferreira LKL, Araújo Lima AL, Barbosa Cordeiro M, Ferreira Costa H, et al. Synthesis, toxicity study and anti-inflammatory effect of MHTP, a new tetrahydrotetrahydroisoquinoline alkaloid. Immunopharmacol Immunotoxicol. 2015;37:400–12.
Paiva Ferreira LKD, Paiva Ferreira LAM, Alves AF, Leite FC, de Araújo Silva LA, Vieira GC, et al. MHTP, 2-METHOXY-4-(7-methoxy-1,2,3,4-tetrahydroisoquinolin-1-yl) phenol, a synthetic alkaloid, induces IFN-γ production in murine model of ovalbumin-induced pulmonary allergic inflammation. Inflammation [Internet]. 2018. http://link.springer.com/10.1007/s10753-018-0855-y.
Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome. Am J Respir Cell Mol Biol [Internet]. 2005 [cited 2019 Mar 19];33:319–27. http://www.ncbi.nlm.nih.gov/pubmed/16172252.
Walkey A, Summer R, Ho V, Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol [Internet]. 2012 [cited 2019 Mar 19];4:159. http://www.ncbi.nlm.nih.gov/pubmed/22866017.
Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet [Internet]. 2007 [cited 2019 Mar 19];369:1553–64. http://www.ncbi.nlm.nih.gov/pubmed/17482987.
Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod [Internet]. 2016 [cited 2019 Mar 19];79:629–61. http://www.ncbi.nlm.nih.gov/pubmed/26852623.
Lahlou M. The success of natural products in drug discovery. Pharmacol Pharm [Internet]. Scientific Research Publishing; 2013 [cited 2019 Mar 19];04:17–31. http://www.scirp.org/journal/doi.aspx?DOI=10.4236/pp.2013.43A003.
D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, et al. CD4+ CD25+ Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest [Internet]. 2009 [cited 2018 Aug 20];119:2898–913. http://www.ncbi.nlm.nih.gov/pubmed/19770521.
Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv [Internet]. Mary Ann Liebert, Inc.; 2010 [cited 2018 Aug 20];23:243–52. http://www.ncbi.nlm.nih.gov/pubmed/20073554.
Cross LJM, Matthay MA. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin [Internet]. 2011 [cited 2019 Mar 19];27:355–77. http://www.ncbi.nlm.nih.gov/pubmed/21440206.
Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med [Internet]. The Feinstein Institute for Medical Research; 2011 [cited 2019 Mar 19];17:293–307. http://www.ncbi.nlm.nih.gov/pubmed/21046059.
Grudzinska FS, Sapey E. Friend or foe? The dual role of neutrophils in lung injury and repair. Thorax [Internet]. 2018 [cited 2019 Mar 19];73:305–7. http://www.ncbi.nlm.nih.gov/pubmed/29382796.
Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol [Internet]. 2004 [cited 2019 Mar 19];202:145–56. http://www.ncbi.nlm.nih.gov/pubmed/14743496.
Bouros D, Alexandrakis MG, Antoniou KM, Agouridakis P, Pneumatikos I, Anevlavis S, et al. The clinical significance of serum and bronchoalveolar lavage inflammatory cytokines in patients at risk for Acute Respiratory Distress Syndrome. BMC Pulm Med [Internet]. 2004 [cited 2019 Mar 19];4:6. http://www.ncbi.nlm.nih.gov/pubmed/15315713.
Marshall RP, Webb S, Hill MR, Humphries SE, Laurent GJ. Genetic polymorphisms associated with susceptibility and outcome in ARDS. Chest [Internet]. 2002 [cited 2019 Mar 19];121:68S–69S. http://www.ncbi.nlm.nih.gov/pubmed/11893690.
Li X, Liu D, Chen H, Pan X, Kong Q, Pang Q. Lactoferrin protects against lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol [Internet]. 2012 [cited 2019 Mar 19];12:460–4. http://www.ncbi.nlm.nih.gov/pubmed/22251871.
Albiger B, Dahlberg S, Henriques-Normark B, Normark S. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med [Internet]. 2007 [cited 2019 Mar 19];261:511–28. http://www.ncbi.nlm.nih.gov/pubmed/17547708.
Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, et al. Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury. Int J Exp Pathol [Internet]. 2007 [cited 2019 Mar 20];88:387–91. http://www.ncbi.nlm.nih.gov/pubmed/18039275.
Yang R, Yang L, Shen X, Cheng W, Zhao B, Ali KH, et al. Suppression of NF-κB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol [Internet]. 2012 [cited 2019 Mar 20];674:391–6. http://www.ncbi.nlm.nih.gov/pubmed/21925167.
Patel B V., Wilson MR, Takata M. Resolution of acute lung injury and inflammation: a translational mouse model. Eur Respir J [Internet]. 2012 [cited 2019 Mar 20];39:1162–70. http://www.ncbi.nlm.nih.gov/pubmed/22005920.
Rosseau S, Hammerl P, Maus U, Walmrath H-D, Schütte H, Grimminger F, et al. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Cell Mol Physiol [Internet]. 2000 [cited 2019 Mar 20];279:L25–35. http://www.ncbi.nlm.nih.gov/pubmed/10893199.
Acknowledgements
The Brazilian government sponsored this study by CNPq and CAPES organizations.
Author information
Authors and Affiliations
Contributions
MRP, BMNX and LAMPF conceived and designed the experiments. BMNX, LAMPF, and LKDPF performed experimental model of acute lung injury. BMNX, LAMPF, LKDPF, and TMM performed ELISA and flow cytometry experiments. LAAS and LCR performed the synthesis of MHTP. BMNX, LAMPF, and FAAFG performed the lung histology. BMNX, LAMPF, LKDPF, and MRP analyzed the data and MRP wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
do Nascimento Xavier, B.M., Ferreira, L.A.M.P., Ferreira, L.K.D.P. et al. MHTP, a synthetic tetratetrahydroisoquinoline alkaloid, attenuates lipopolysaccharide-induced acute lung injury via p38MAPK/p65NF-κB signaling pathway-TLR4 dependent. Inflamm. Res. 68, 1061–1070 (2019). https://doi.org/10.1007/s00011-019-01291-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01291-3