Abstract
Background
Kaempferol-3-O-sophoroside (KPOS) was isolated from the leaves of cultivated mountain ginseng. Kaempferol (KP) has antitumor, anti-oxidative, anti-allergic and antidiabetic activities but the barrier protective effects and underlying mechanism are not fully identified. In this study, we attempted to determine whether pretreatment with KPOS induced significant barrier protective activities in lipopolysaccharide (LPS)-stimulated human umbilical vein endothelial cells (HUVECs).
Methods
The anti-inflammatory activities of KPOS were determined by measuring solute flux, neutrophil adhesion and migration and activation of pro-inflammatory proteins in LPS-activated HUVECs.
Results
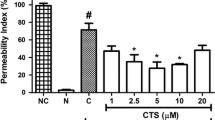
We found that KPOS inhibited LPS-induced barrier disruption, expression of cell adhesion molecules, neutrophil adhesion and transendothelial migration of neutrophils to HUVECs. Further studies revealed that KPOS suppressed the production of tumor necrosis factor-α (TNF-α) and activation of nuclear factor-κB (NF-κB) by LPS, and that anti-inflammatory activities of KPOS were better than those of KP.
Conclusion
Collectively, these results suggest that KPOS possesses barrier integrity activity, inhibitory activity on cell adhesion and migration to endothelial cells by blocking the activation of NF-κB expression and production of TNF-α, thereby endorsing its usefulness as therapy for vascular inflammatory diseases.





Similar content being viewed by others
Abbreviations
- KP:
-
Kaempferol
- KPOS:
-
Kaempferol-3-O-sophoroside
- LPS:
-
Lipopolysaccharide
- CAM:
-
Cell adhesion molecule
- VCAM:
-
Vascular cell adhesion molecule
- ICAM:
-
Intracellular cell adhesion molecule
- NF-κB:
-
Nuclear factor-κB
- TNF:
-
Tumor necrosis factor
- BDMC:
-
Bisdemethoxycurcumin
- TEM:
-
Transendothelial migration
References
Keller TT, Mairuhu AT, de Kruif MD, Klein SK, Gerdes VE, ten Cate H, et al. Infections and endothelial cells. Cardiovasc Res. 2003;60:40–8.
Javaid K, Rahman A, Anwar KN, Frey RS, Minshall RD, Malik AB. Tumor necrosis factor-alpha induces early-onset endothelial adhesivity by protein kinase Czeta-dependent activation of intercellular adhesion molecule-1. Circ Res. 2003;92:1089–97.
Lockyer JM, Colladay JS, Alperin-Lea WL, Hammond T, Buda AJ. Inhibition of nuclear factor-kappaB-mediated adhesion molecule expression in human endothelial cells. Circ Res. 1998;82:314–20.
Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, et al. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993;92:1866–74.
Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–42.
Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–72.
Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm Des. 2006;12:4229–45.
Wung BS, Ni CW, Wang DL. ICAM-1 induction by TNF-alpha and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J Biomed Sci. 2005;12:91–101.
Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–19.
Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003;83:1069–112.
Bae JS, Yang L, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J Biol Chem. 2007;282:9251–9.
Spiecker M, Darius H, Liao JK. A functional role of I kappa B-epsilon in endothelial cell activation. J Immunol. 2000;164:3316–22.
Takeda R, Suzuki E, Satonaka H, Oba S, Nishimatsu H, Omata M, et al. Blockade of endogenous cytokines mitigates neointimal formation in obese Zucker rats. Circulation. 2005;111:1398–406.
Olszewska M. Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. J Pharm Biomed Anal. 2008;48:629–35.
Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of kaempferol on the production and gene expression of monocyte chemoattractant protein-1 in J774.2 macrophages. Pharmacol Rep. 2005;57:107–12.
Garcia-Mediavilla V, Crespo I, Collado PS, Esteller A, Sanchez-Campos S, Tunon MJ, et al. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol. 2007;557:221–9.
Zhu M, Chan KW, Ng LS, Chang Q, Chang S, Li RC. Possible influences of ginseng on the pharmacokinetics and pharmacodynamics of warfarin in rats. J Pharm Pharmacol. 1999;51:175–80.
Choo MK, Park EK, Han MJ, Kim DH. Antiallergic activity of ginseng and its ginsenosides. Planta Med. 2003;69:518–22.
Dey L, Zhang L, Yuan CS. Anti-diabetic and anti-obese effects of ginseng berry extract: comparison between intraperitoneal and oral administrations. Am J Chin Med. 2002;30:645–7.
Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC, Sun YX. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or pro-oxidative activity in free radical induced hemolysis of human erythrocytes. J Agric Food Chem. 2003;51:2555–8.
Schliemann W, Schneider B, Wray V, Schmidt J, Nimtz M, Porzel A, et al. Flavonols and an indole alkaloid skeleton bearing identical acylated glycosidic groups from yellow petals of Papaver nudicaule. Phytochemistry. 2006;67:191–201.
Bae JS, Kim YU, Park MK, Rezaie AR. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 Kinase. J Cell Physiol. 2009;219:744–51.
Bae JS, Rezaie AR. Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand. Thromb Haemost. 2008;100:101–9.
Akeson AL, Woods CW. A fluorometric assay for the quantitation of cell adherence to endothelial cells. J Immunol Methods. 1993;163:181–5.
Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–20.
Che W, Lerner-Marmarosh N, Huang Q, Osawa M, Ohta S, Yoshizumi M, et al. Insulin-like growth factor-1 enhances inflammatory responses in endothelial cells: role of Gab1 and MEKK3 in TNF-alpha-induced c-Jun and NF-kappaB activation and adhesion molecule expression. Circ Res. 2002;90:1222–30.
Fisher M. Injuries to the vascular endothelium: vascular wall and endothelial dysfunction. Rev Neurol Dis. 2008;5(Suppl 1):S4–11.
Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003;24:640–7.
Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81.
De Nardin E. The role of inflammatory and immunological mediators in periodontitis and cardiovascular disease. Ann Periodontol. 2001;6:30–40.
Hopkins SJ. The pathophysiological role of cytokines. Leg Med (Tokyo). 2003;5(Suppl 1):S45–57.
Lacasse C, Turcotte S, Gingras D, Stankova J, Rola-Pleszczynski M. Platelet-activating factor stimulates interleukin-6 production by human endothelial cells and synergizes with tumor necrosis factor for enhanced production of granulocyte-macrophage colony stimulating factor. Inflammation. 1997;21:145–58.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government [MEST] (No. 2011-003410, 2011-0026695, 2011-0030124).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Liwu Li.
T. H. Kim, S.-K. Ku and I.-C. Lee contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, T.H., Ku, SK., Lee, IC. et al. Anti-inflammatory effects of kaempferol-3-O-sophoroside in human endothelial cells. Inflamm. Res. 61, 217–224 (2012). https://doi.org/10.1007/s00011-011-0403-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0403-9