Abstract
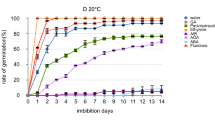
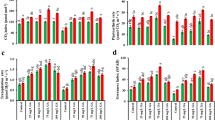
Germination of tomato cv. New Yorker seed is inhibited at 35°C. This thermoinhibition was partially counteracted by application of GA4+7 alone, the compound applied in combination with ACC or ethephon markedly enhancing the process. The latter compound alone was not able to induce germination at 35 °C. Thermoinhibition of seeds at 35 °C was also counteracted by fluridone, an inhibitor of ABA biosynthesis. At 25 °C, an optimal temperature, ABA inhibited germination of New Yorker seeds. Although another known growth inhibitor MeJA, when applied at an optimal temperature (25 °C), had also a slightly inhibitory effect on germination of those seeds and clearly delayed the process, inhibitors of its biosynthetic pathway (ibuprofen, indoprofen, antypiryne and salicylic acid) did not remove thermoinhibition at 35 °C. An increase in endo-β-mannanase activity after 24 hours of incubation at 35 °C was observed in the seeds incubated in the presence of gibberellins, ACC, ethephon, fluridone used alone and in combinations, but it was not clearly correlated with the effects of these compounds on alleviation of seed germination. However, fluridone present in the same incubation medium at 35 °C with ABA was able to counteract the inhibitory effect of ABA on endo-β-mannanase activity.
The results of our study suggest that gibberellins, ethylene (produced from ACC or ethephon) and ABA, but not jasmonates, regulate tomato seed germination at supraoptimal temperatures. Alleviation of thermoinhibition of New Yorker seed germination by plant growth regulators and fluridone is partially associated with their controlling endo-β-mannanase activity.
Similar content being viewed by others
Abbreviations
- ABA:
-
abscisic acid
- ACC:
-
1-aminocyclopropane-1-carboxylic acid
- E:
-
ethephon
- F:
-
fluridone
- GA4+7 :
-
gibberellins A4+7
- MeJA:
-
methyl jasmonate
References
Bewley J.D., Black M. 1994. Seeds. Physiology of development and germination. 2nd ed. Plenum Press. New York.
Bewley J.D. 1997. Seed germination and dormancy. Plant Cell, 9: 1055–1056.
Corbineau F., Rudnicki R.M., Come D. 1988. The effect of methyl jasmonate on sunflower (Helianthus annuus L.) seed germination and seedling development. Plant Growth Regul., 7: 157–169.
Creelman R.A., Mullet J.E. 1995. Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc. Nat. Acad. Sci. USA., 92: 4114–4119.
Downie B.H., Hilhorst W.M., Bewley J.D. 1994. A new assay for quantifying endo-β-mannanase activity using Congo Red dye. Phytochemistry, 36: 829–835.
Dutta S., Bradford K.J. 1994. Water relations of lettuce seed thermoinhibition. II. Ethylene and endosperm effects on base water potential. Seed Sci. Res., 4: 11–18.
Gonai T., Kawahara S., Tougou M., Satoh S., Iashiba T., Hirai N., Kawaide H., Kamiya Y., Yoshioka T. 2004. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J. Exp. Bot., 55: 111–118.
Grappin P., Bouinot D., Sotta B., Miginiac E., Jullien M. 2000. Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta, 210: 279–285.
Groot S.P.C., Kaliszewska-Rokicka B., Vermeer E., Karssen C.M. 1988. Gibberellin-induced hydrolysis of endosperm cell walls in gibberellin-deficient tomato seeds prior to radicle protrusion. Planta, 174: 500–504.
Groot S.P.C., Karssen C.M. 1987. Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellins-deficient mutants. Planta, 171: 25–31.
Groot S.P.C., Karssen C.M. 1992. Dormancy and germination of abscisic acid-deficient tomato seeds. Studies with the silens mutant. Plant Physiol., 99: 952–958.
Harms K., Ramirez I., Pe a-Cortés H. 1998. Inhibition of wound-induced accumulation of allene oxide synthase transcripts in flax leaves by aspirin and salicylic acid. Plant Physiol., 118: 1057–1065.
Hung K.T., Kao C.H. 1996. Promotive effect of jasmonates on the senescence of detached maize leaves. Plant Growth Regul., 19: 77–83.
Kpczyski J. 1986. Inhibition of Amaranthus caudatus seed germination by polyethylene glycol-6000 and abscisic acid and its reversal by ethephon or 1-aminocyclo-1-carboxylic acid. Physiol. Plant., 67: 588–591.
Kpczyski J., Białecka B. 1994. Stimulatory effect of ethephon, ACC, gibberellin A3 and A4+7 on germination of methyl jasmonate inhibited Amaranthus caudatus L. seeds. Plant Growth Regul., 14: 211–216.
Kpczyski J., Bihun M., K pczy ska E. 2003. The release of secondary dormancy by ethylene in Amaranthus caudatus L. seeds. Seed Sci. Res., 13: 69–74.
Mo B., Bewley J.D. 2003. Mobilization of the galactomannan-containing cell wall of tomato seeds: where does β-mannosidase fit into the picture? In: The Biology of Seeds. Recent Research Advances, ed. by Nicolas G., Bradford K.J., Come D., Pritchard H.W., CAB International, Wallingfort, UK: 121–129.
Nascimento W.M. 2003. Ethylene and lettuce seed germination. Sci. Agric., 60: 601–606.
Nomaguchi M., Nonogaki H., Morohashi Y. 1995. Development of galactomannan, hydrolyzing activity in the micropylar endosperm tip of tomato seed prior to germination. Physiol. Plant., 94: 105–109.
Nonogaki H., Nomaguchi M., Okumoto N., Kaneko Y., Matsushima H., Morohashi Y. 1998. Temporal and spatial pattern of the biochemical activation of the endosperm during and following imbition of tomato seeds. Physiol. Plant., 102: 236–242.
Nonogaki H., Gee O.H., Bradford K.J. 2000. A germination specific endo-β-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol., 123: 1235–1246.
Saini S.H., Consolacion E.V., Bassi, P.K., Spencer M.S. 1986. Requirement for ethylene synthesis and action during relief of thermoinhibition of lettuce seed germination by combinations of gibberellic acid, kinetin and carbon dioxide. Plant Physiol., 81: 950–953.
Saini S.H., Consolacion E.V., Bassi P.K., Spencer M.S. 1989. Control processes in the induction and relief of thermoinhibition of lettuce seed germination. Plant Physiol., 90: 311–315.
Steward C.R., Voetberg G. 1987. Abscisic acid accumulation is not required for proline accumulation in wilted leaves. Plant Physiol., 83: 747–749.
Still D.W., Bradford K.J. 1997. A single-seed assay for endo-β-mannanase activity from tomato endosperm and radicle tissues. Plant Physiol., 113: 13–20.
Toorop P.E., van Aelst A.C., Hilhorst H.W.M. 1998. Endosperm cap weakening and endo-β-mannanase activity during priming of tomato (Lycopersicon esculentum cv. Moneymaker) seeds are initiated upon a threshold water potential. Seed Sci. Res., 8: 483–491.
Vidaver W., Hsiao A.I. 1975. Action of gibberrelic acid and phytochrome on the germination of Grand Rapids lettuce seeds. Plant Physiol., 53: 266–268.
Voigt B., Bewley J.D. 1996. Developing tomato seeds when removed from the fruit produce multiple forms of germinative and post-germinative endo-β-mannanase. Response to desiccation, abscisic acid and osmoticum. Planta, 200: 71–77.
Yoshioka T., Endo T., Satoh S. 1998. Restoration of seed germination at supraoptimal temperatures by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol., 39: 307–312.
Yoshioka T., Gonai T., Kawahara S., Satoh S., Hashiba T. 2003. The regulation of the thermoinhibition of seed germination in winter annual plants by abscisic acid. In: The Biology of Seeds. Recent Research Advances, ed. by Nicolas G., Bradford K.J., Come D., Pritchard H.W., CAB International, Wallingfort, UK: 217–223.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kpczyska, E., Pikna-Grochala, J. & Kpczyski, J. Hormonal regulation of tomato seed germination at a supraoptimal temperature. Acta Physiol Plant 28, 225–231 (2006). https://doi.org/10.1007/BF02706534
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02706534