Abstract
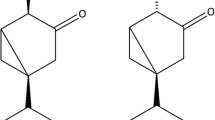
Two sesquiterpene hydrocarbons, β-copaene and β-ylangene, were isolated from bioactive fractions of angelica seed oil and were shown by field bioassays to be attractive to the male Mediterranean fruit fly. Their relative attractiveness, compared with the(+)-and (−)-α-copaene enantiomers, are: (+)-α-copaene>angelica β-copaene>angelica β-ylangene>(−)-α-copaene. The enantiomer ratios for the two compounds are: β-copaene, 61.4% (+), 38.6% (−); β-ylangene, 91.9% (+), 8.1% (−).trans-α-Bergamotene was also isolated from the same fractions, but in sufficient quantity for bioassay [enantiomer ratio: 95.7% (+), 4.3% (−)].
Similar content being viewed by others
References
Cochran, W.G., andCox, G.M. 1957. Experimental Designs, 2nd ed. J. Wiley & Sons, New York.
Corey, E.J., andWatt, D.S. 1973. A total synthesis of (±)-α and (±)-β-copaenes and ylangenes.J. Amer. Chem. Soc. 95:2303–2311.
DeMayo, P., Williams, R.E., Büchi, G., andFeairheller, S.H. 1965. The absolute stereo-structure of copaene.Tetrahedron 21:619–627.
Fornasiero, U., Guiotto, A., Caporale, G., Baccichetti, F., andMusajo, L. 1969. Identificazione della sostanza attrattiva per i maschi dellaCeratitis capitata, contenunuta nell'olio essenziale dei semi diAngelica archAngelica.Gazz. Chim. Ital. 99:700–710.
Guiotto, A., Fornasiero, U., andBaccichetti, F. 1972. Investigations on attractants for males ofCeratitis capitata.II Farmaco 27:663–669.
Harris E.J., Nakagawa, S., andUrago, T. 1971. Sticky traps for detection and survey of three tephritids.J. Econ. Entomol. 64:62–65.
Heathcock, C.H. 1966. The total synthesis of (±)-copaene and (±)-8-isocopaene.J. Am. Chem. Soc. 88:4110–4112.
Heathcock, C.H., Badger, R.A., andPatterson, J.W., Jr. 1967. Total synthesis of (±)-copaene and (±)-ylangene. A general method for the synthesis of tricyclo[4.4.0.02,7]decanes.J. Am. Chem. Soc. 89:4133–4145.
Hirose, Y. 1967. Mass spectra of sesquiterpenes.Shitsuryo Bunseki 15:162–178.
Hunter, G.L.K., andBrogden, W.B., Jr. 1964.β-Ylangene, a new sesquiterpene hydrocarbon from orange oil.J. Org. Chem. 29:2100.
Jacobson, M., Uebel, E.C., Lusby, W.R., andWaters, R.M. 1987. Optical isomers ofα-copaene derived from several plant sources.J. Agric. Food Chem. 35:798–800.
Kapadia, V.H., Nagasampagi, B.A., Naik, V.G., andDev, S. 1965. Studies in sesquiterpenes—XXII. Structure of mustakone and copaene.Tetrahedron 21:607–618.
Krishnappa, S., andDev, S. 1973. Sesquiterpenes fromLansium anamalayanum.Phytochemistry 12:823–825.
Motl, O., Bucharov, V., Herout, V., andŠorm, F. 1963. The structure of ylangene—a new type of tricyclic sesquiterpene.Chem Ind., 1759–1760.
Motl, O., Herout, V., andŠorm, F. 1965. Structure of the sesquiterpenic hydrocarbon ylangene.Tetrahedron Lett., 451–455.
Ohta, Y., andHirose, Y. 1969. Stereochemistry of (+)α-ylangene.Tetrahedron Lett., 1601–1604.
Ohta, Y., Ohara, K., andHirose, Y. 1968. Acid catalyzed isomerization ofα-cubebene,α-copaene andα-ylangene.Tetrahedron Lett., 4181–4184.
Schreier, P., Drawert, F., andJunker, A. 1976. Identification of volatile constituents from grapes.J. Agric. Food Chem. 24:331–336.
Steiner, L.F., Miyashita, D.H., andChristenson, L.D. 1957. Angelica oils as Mediterranean fruit fly lures.J. Econ. Entomol. 50:505.
Takeoka, G., Flath, R.A., Mon, T.R., Buttery, R.G., Teranishi, R., Güntert, M., Lautamo, R., andSzejtli, J. 1990. Further applications of permethylatedβ-cyclodextrin capillary gas chromatographic columns. HRC & CC. 13:202–206.
Taskinen, J., andNykänen, L. 1975. Chemical composition of Angelica root oil.Acta Chem. Scand. B. 29:757–764.
Waller, R.A., andDuncan, D.B. 1969. A Bayes rule for symmetrical multiple comparisons problem.J. Am. Stat. Assoc. 64:1484–1503.
Warthen, J.D., Jr., andMcInnis, D.O. 1989. Isolation and identification of male medfly attractive components inLitchi chinensis stems andFicus spp. stem exudates.J. Chem. Ecol. 15:1931–1946.
Wenninger, J.A., andYates, R.L. 1970. High resolution infrared spectra of some naturally occurring sesquiterpene hydrocarbons. II. Second series. JAOAC 53:949–963.
Wenninger, J.A., Yates, R.L., andDolinsky, M. 1967. High resolution infrared spectra of some naturally occurring sesquiterpene hydrocarbons. JAOAC 50:1313–1335.
Westfelt, L. 1966. High-boiling constituents of Swedish sulphate turpentine (Pinus silvestris L.)Acta Chem. Scand. 20:2841–2851.
Westfelt, L. 1967.β-Copaene andβ-ylangene, minor sesquiterpenes of the wood ofPinus silvestris L. and of Swedish sulphate turpentine.Acta Chem. Scand. 21:152–158.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Flath, R.A., Cunningham, R.T., Mon, T.R. et al. Additional male mediterranean fruitfly (Ceratitis capitata wied.) Attractants from Angelica seed oil (Angelica archangelica L.). J Chem Ecol 20, 1969–1984 (1994). https://doi.org/10.1007/BF02066237
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02066237