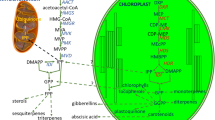
Abstract
The net value of any plant trait can be assessed by measuring the costs and benefits associated with that trait. While the other contributors to this issue examine the possible benefits of terpenoids to plants, this article explores the metabolic costs of terpenoid accumulation in plants in the light of recent advances in terpenoid biochemistry. Terpenoids are more expensive to manufacture per gram than most other primary and secondary metabolites due to their extensive chemical reduction. The enzyme costs of making terpenoids are also high since terpenoid biosynthetic enzymes are apparently not shared with other metabolic pathways. In fact, plant cells may even possess more than one set of enzymes for catalyzing the basic steps of terpenoid formation. Terpenoids are usually sequestered in complex, multicellular secretory structures, and so storage costs for these substances are also likely to be substantial. However, not all of the processes involved in terpenoid accumulation require large investments of resources. For instance, the maintenance of terpenoid pools is probably inexpensive because there is no evidence that substantial quantities of terpenes are lost as a result of metabolic turnover, volatilization, or leaching. Moreover, plants may reduce their net terpenoid costs by employing individual compounds in more than one role or by catabolizing substances that are no longer needed, although it is still unclear if such practices are widespread. These findings (and other facets of terpenoid biochemistry and physiology) are discussed in relation to the assumptions and predictions of several current theories of plant defense, including the carbonnutrient balance hypothesis, the growth-differentiation balance hypothesis, and the resource availability hypothesis.
Similar content being viewed by others
References
Adams, R.P., andHagerman, A. 1977. Diurnal variation in the volatile terpenoids ofJuniperus scopulorum (Cupressaceae).Am. J. Bot. 64:278–285.
Adzet, T., Ponz, R., Wolf, E., andSchulte, E. 1992. Genetic variability of the essential oil content ofMelissa officinalis.Planta Med. 58:558–561.
Alonso, W.R., andCroteau, R. 1991. Purification and characterization of the monoterpene cyclaseγ-terpinene synthase fromThymus vulgaris.Arch. Biochem. Biophys. 286:511–517.
Alonso, W.R., Rajaonarivony, J.I.M., Gershenzon, J., andCroteau, R. 1992. Purification of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha × piperita) and spearmint (Mentha spicata).J. Biol. Chem. 267:7582–7587.
Amelunxen, F. 1965. Elektronenmikroskopische Untersuchungen an den Drüsenschuppen vonMentha piperita L.Planta Med. 13:457–473.
Asakawa, Y., Dawson, G.W., Griffiths, D.C., Lallemand, J.-Y., Ley, S. V., Mori, K., Mudd, A., Pezechk-Leclaire, M., Pickett, J.A., Watanabe, H., Woodcock, C.M., andZhong-Ning, Z. 1988. Activity of drimane antifeedants and related compounds against aphids, and comparative biological effects and chemical reactivity of (-)- and (+)-polygodial.J. Chem. Ecol. 14:1845–1855.
Bach, T.J. 1986. Hydroxymethylglutaryl-CoA reductase, a key enzyme in phytosterol synthesis?Lipids 21:82–88.
Bach, T.J., Boronat, A., Caelles, C., Ferrer, A., Weber, T., andWettstein, A. 1991. Aspects related to mevalonate biosynthesis in plants.Lipids 26:637–648.
Bakker, M.E., andBaas, P. 1993. Cell walls in oil and mucilage cells.Acta Bot. Neerl. 42:133–139.
Bakker, M.E., Gerritsen, A.G., andVan Der Schaff, P.J. (1991). Development of oil and mucilage cells inCinnamomum burmanni. An ultrastructural study.Acta Bot. Neerl. 40:339–356.
Baldwin, I.T., Sims, C.L., andKean, S.E. 1990. The reproductive consequences associated with inducible alkaloidal responses in wild tobacco.Ecology 71:252–262.
Balliano, G., Caputo, O., Viola, F., Delprino, L., andCattel, L. 1983. The transformation of 10α-cucurbita-5,24-dien-3β-ol into cucurbitacin C by seedlings ofCucumis sativus.Phytochemistry 22:909–913.
Banthorpe, D.V., andEkundayo, O. 1976. Biosynthesis of (+)-car-3-ene inPinus species.Phytochemistry 15:109–112.
Banthorpe, D.V., Doonan, H.J., andWirz-Justice, A. 1972. Terpene biosynthesis. Part V. Interconversions of some monoterpenes in higher plants and their possible role as precursors of carotenoids.J. Chem. Soc. Perkin Trans. I, 1972:1764–1769.
Belingheri, L., Pauly, G., Gleizes, M., andMarpeau, A. 1988. Isolation by aqueous two-polymer phase system and identification of endomembranes fromCitrofortunella mitis fruits for sesquiterpene hydrocarbon synthesis.J. Plant Physiol. 132:80–85.
Berger., R.G., Akkan, Z., andDrawert, R. 1990. Catabolism of geraniol by cell suspension cultures ofCitrus limon.Biochim. Biophys. Acta 1055:234–239.
Bernard-Dagan, C. 1988. Biosynthesis of lower terpenoids: Genetic and physiological controls in woody plants, pp. 329–351,in J.W. Hanover and D.E. Keathley (eds.). Genetic Manipulation of Woody Plants. Plenum Press, New York.
Bernard-Dagan, C., Pauly, G., Marpeau, A., Gleizes, M., Carde, J.-P., andBaradat, P. 1982. Control and compartmentation of terpene biosynthesis in leaves ofPinus pinaster.Physiol. Veg. 20:775–795.
Bhatt, J.R. 1987. Development and structure of primary secretory ducts in the stem ofCommiphora wightii (Burseraceae).Ann. Bot. 60:405–416.
Bjorkman, C., Larsson, S., andGref, R. 1991. Effects of nitrogen fertilization on pine needle chemistry and sawfly performance.Oecologica 86:202–209.
Breccia, A., andBadiello, R. 1967. The role of general metabolites in the biosynthesis of natural products. I. The terpene marrubiin.Z. Naturforsch. 22b:44–49.
Briggs, M.A., andSchultz, J.C. 1990. Chemical defense production inLotus corniculatus L. II. Trade-offs among growth, reproduction and defense.Oecologia 83:32–37.
Brown, D.G. 1988. The cost of plant defense: An experimental analysis with inducible proteinase inhibitors in tomato.Oecologia 76:467–470.
Bryant, J.P., Chapin, F.S., III, andKlein, D.R. 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory.Oikos 40:357–368.
Bryant, J.P., Reichardt, P.B., Clausen, T.P., Provenza, F.D., andKuropat, P.J. 1992. Woody plant-mammal interactions, pp. 343–370,in G.A. Rosenthal and M.R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites, Vol. 2, 2nd ed. Academic Press, San Diego.
Burbott, A.J., andLoomis, W.D. 1969. Evidence for metabolic turnover of monoterpenes in peppermint.Plant Physiol. 44:173–179.
Caelles, C., Ferrer, A., Balcells, L., Hegardt, F.G., andBoronat, A. 1989. Isolation and structural characterization of a cDNA encodingArabidopsis thaliana 3-hydroxy-3-methylglutaryl coenzyme A reductase.Plant Mol. Biol. 13:627–638.
Cane, D.E. 1990. Enzymatic formation of sesquiterpenes.Chem. Rev. 90:1089–1103.
Carlton, R.R., Waterman, P.G., andGray, A.I. 1992. Variation of leaf gland volatile oil within a population of sweet gale (Myrica gale) (Myricaceae).Chemoecology 3:45–54.
Chapin, F.S., III. 1980. The mineral nutrition of wild plants.Annu. Rev. Ecol. Syst. 11:233–260.
Chapin, F.S., III. 1989. The cost of tundra plant structures: Evaluation of some concepts and currencies.Am. Nat. 133:1–19.
Chappell, J., Vonlanken, C., andVogeli, U. 1991. Elicitor-inducible 3-hydroxy-3-methylglutaryl coenzyme A reductase activity is required for sesquiterpene accumulation in tobacco cell suspension cultures.Plant Physiol. 97:693–698.
Charon, J., Launay, J., andVindt-Balguerie, E. 1986. Ontogenèse des canaux sécréteurs d'origine primaire dans le bourgeon de Pin maritime.Can. J. Bot. 64:2955–2964.
Chew, F.S., andRodman, J.E. 1979. Plant resources for chemical defense, pp. 271–307,in G.A. Rosenthal and D.H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites, 1st ed. Academic Press, New York.
Chiariello, N.R., Mooney, H.A., andWilliams, K. 1989. Growth, carbon allocation and cost of plant tissues, pp. 327–365,in R.W. Pearcy, J.R. Ehleringer, H.A. Mooney, and P.W. Rundel (eds.). Plant Physiological Ecology: Field Methods and Instrumentation. Chapman and Hall, London.
Chye, M.-L., Tan, C.-T., andChua, N.-H. 1992. Three genes encode 3-hydroxy-3-methylglutaryl-coenzyme A reductase inHevea brasiliensis: hmg1 andhmg3 are differentially expressed.Plant Mol. Biol. 19:473–484.
Coley, P.D., Bryant, J.P., andChapin, F.S., III. 1985. Resource availability and plant antiherbivore defense.Science 230:895–899.
Cornish, K. 1992. Natural rubber biosynthesis, a branch of the isoprenoid pathway in plants, pp. 18–26,in W.D. Nes, E.J. Parish, and J.M. Trzaskos (eds.). Regulation of Isopentenoid Metabolism, ACS Symposium Series No. 497. American Chemical Society, Washington, D.C.
Cotton, C.M., Evans, L.V., andGramshaw, J.W. 1991. The accumulation of volatile oils in whole plants and cell cultures of tarragon (Artemisia dracunculus).J. Exp. Bot. 42:365–375.
Croteau, R. 1987. Biosynthesis and catabolism of monoterpenoids.Chem. Rev. 87:929–954.
Croteau, R. 1988. Catabolism of monoterpenes in essential oil plants, pp. 65–84,in B.D. Mookherjee and B.J. Willis (eds.). Flavors and Fragrances: A World Perspective. Elsevier, Amsterdam.
Croteau, R., andGershenzon, J. 1994. Genetic control of monoterpene biosynthesis in mints (Mentha: Lamiaceae),in H. Stafford (ed.). Recent Advances in Phytochemistry, Vol. 28. Plenum Press, New York.
Croteau, R., andLoomis, W.D. 1972. Biosynthesis of mono- and sesquiterpenes in peppermint from mevalonate-2-14C.Phytochemistry 11:1055–1066.
Croteau, R., andLoomis, W.D. 1973. Biosynthesis of squalene and other triterpenes inMentha piperita from mevalonate-2-14C.Phytochemistry 12:1957–1965.
Croteau, R., andShaskus, J. 1985. Biosynthesis of monoterpenes: Demonstration of a geranyl pyrophosphate: (-)-bornyl pyrophosphate cyclase in soluble enzyme preparations from tansy (Tanacetum vulgare).Arch. Biochem. Biophys. 236:535–543.
Croteau, R., andSood, V.K. 1985. Metabolism of monoterpenes: Evidence for the function of monoterpene catabolism in peppermint (Mentha piperita) rhizomes.Plant Physiol. 77:801–806.
Croteau, R., andVenkatachalam, K.V. 1986. Metabolism of monoterpenes: Demonstration that (+)-cis-isopulegone, not piperitenone, is the key intermediate in the conversion of (-)-isopiperitenone to (+)-pulegone in peppermint (Mentha piperita).Arch. Biochem. Biophys. 249:306–315.
Croteau, R., andWinters, J.N. 1982. Demonstration of the intercellular compartmentation ofl-menthone metabolism in peppermint (Mentha piperita) leaves.Plant Physiol. 69:975–977.
Croteau, R., Burbott, A.J., andLoomis, W.D. 1972a. Biosynthesis of mono- and sesqui-terpenes in peppermint from glucose-14C and14CO2.Phytochemistry 11:2459–2467.
Croteau, R., Burbott, A.J., andLoomis, W.D. 1972b. Apparent energy deficiency in mono- and sesqui-terpene biosynthesis in peppermint.Phytochemistry 11:2937–2948.
Croteau, R., Felton, M., Karp, F., andKjonaas, R. 1981. Relationship of camphor biosynthesis to leaf development in sage (Salvia officinalis).Plant Physiol. 67:820–824.
Croteau, R., Sood, V.K., Renstrom, B., andBhusan, R. 1984a. Metabolism of monoterpenes: Early steps in the metabolism ofd-neomenthyl-β-d-glucoside in peppermint (Mentha piperita) rhizomes.Plant Physiol. 76:647–653.
Croteau, R., El-Bialy, H., andEl-Hindawi, S. 1984b. Metabolism of monoterpenes: Lactonization of (+)-camphor and conversion of the corresponding hydroxy acid to the glucosideglucose ester in sage (Salvia officinalis).Arch. Biochem. Biophys. 228:667–680.
Croteau, R., Munck, S.L., Akoh, C.C., Fisk, H.J., andSatterwhite, D.M. 1987a. Biosynthesis of the sesquiterpene patchoulol from farnesyl pyrophosphate in leaf extracts ofPogostemon cablin (patchouli): Mechanistic considerations.Arch. Biochem. Biophys. 256:56–68.
Croteau, R., Gurkewitz, S., Johnson, M.A., andFisk, H.J. 1987b. Biochemistry of oleoresinosis: Monoterpene and diterpene biosynthesis in lodgepole pine saplings infected withCeratocystis clavigera or treated with carbohydrate elicitors.Plant Physiol. 85:1123–1128.
Croteau, R.B., Wheeler, C.J., Cane, D.E., Ebert, R., andHa, H.-J. 1987c. Isotopically sensitive branching in the formation of cyclic monoterpenes: Proof that (-)-α-pinene and (-)-β-pinene are synthesized by the same monoterpene cyclase via deprotonation of a common intermediate.Biochemistry 26:5383–5389.
Croteau, R., El-Bialy, H., andDehal, S.S. 1987d. Metabolism of monoterpenes: Metabolic fate of (+)-camphor in sage (Salvia officinalis).Plant Physiol. 84:649–653.
Damtoft, S., Jensen, S.R., andJessen, C.U. 1993a. Intermediates between 8-epi-deoxyloganic acid and 6,10-dideoxyaucubin in the biosynthesis of antirrhinoside.Phytochemistry 33:1087–1088.
Damtoft, S., Jensen, S.R., Jessen, C.U., andKnudsen, T.B. 1993b. Late stages in the biosynthesis of aucubin inScrophularia.Phytochemistry 33:1089–1093.
DeFaÿ, E. andJacob, J.-L. 1989. Anatomical organization of the laticiferous system in the bark, pp. 3–14,in J. d'Auzac, J.-L. Jacob, and H. Chrestin (eds.). Physiology of Rubber Tree Latex, The Laticiferous Cell and Latex—A Model of Cytoplasm. CRC Press, Boca Raton, Florida.
Dehal, S.S., andCroteau, R. 1987. Metabolism of monoterpenes: Specificity of the dehydrogenases responsible for the biosynthesis of camphor, 3-thujone, and 3-isothujone.Arch. Biochem. Biophys. 258:287–291.
Dehal, S.S., andCroteau, R. 1988. Partial purification and characterization of two sesquiterpene cyclases from sage (Salvia officinalis) which catalyze the respective conversion of farnesyl pyrophosphate to humulene and caryophyllene.Arch. Biochem. Biophys. 261:346–356.
Deighton, N., Glidewell, S.M., Deans, S.G., andGoodman, B.A. 1993. Identification by EPR spectroscopy of carvacrol and thymol as the major sources of free radicals in the oxidation of plant essential oils.J. Sci. Food Agric. 63:221–225.
Dell, B., andMcComb, A.J. 1975. Glandular hairs, resin production, and habitat ofNewcastelia viscida E. Pritzel (Dicrastylidaceae).Aust. J. Bot. 23:373–390.
Dell, B., andMcComb, A.J. 1977. Glandular hair formation and resin secretion inEremophila fraseri F. Meull (Myoporaceae).Protoplasma 92:71–86.
Dell, B., andMcComb, A.J. 1978a. Plant resins—their formation, secretion and possible functions, pp. 277–316,in H.W. Woolhouse (ed.). Advances in Botanical Research, Vol. 6. Academic Press, London.
Dell, B., andMcComb, A.J. 1978b. Biosynthesis of resin terpenes in leaves and glandular hairs ofNewcastelia viscida.J. Exp. Bot. 29:89–95.
Dolman, D.M., Knight, D.W., Salan, U., andToplis, D. 1992. A quantitative method for the estimation of parthenolide and other sesquiterpene lactones containing α-methylenebutyrolactone functions present in feverfew,Tanacetum parthenium.Phytochem. Anal. 3:26–31.
Dudai, N., Putievsky, E., Ravid, U., Palevitch, D., andHalevy, A.H. 1992. Monoterpene content inOriganum syriacum as affected by environmental conditions and flowering.Physiol. Plant. 84:453–459.
Dudley, M.W., Dueber, M.T., andWest, C.A. 1986. Biosynthesis of the macrocyclic diterpene casbene in castor bean (Ricinus communis L.) seedlings: Changes in enzymes levels induced by fungal infection and intracellular localization of the pathway.Plant Physiol. 81:335–342.
Dueber, M.T., Adolf, W., andWest, C.A. 1978. Biosynthesis of the diterpene phytoalexin casbene: Partial purification and characterization of casbene synthetase fromRicinus communis.Plant Physiol. 62:598–603.
Dussourd, D.E., andDenno, R.F. 1991. Deactivation of plant defense: Correspondence between insect behavior and secretory canal architecture.Ecology 72:1383–1396.
Emongor, V.E., andChweya, J.A. 1992. Effect of nitrogen and variety on essential-oil yield and composition from chamomile flowers.Trop. Agric. 60:290–292.
Evans, F.J., andSoper, C.J. 1978. The tigliane, daphnane and ingenane diterpenes, their chemistry, distribution and biological activities. A reviewLloydia 41:193–233.
Fagerstrom, T. 1989. Anti-herbivory chemical defense in plants: A note on the concept of cost.Am. Nat. 133:281–287.
Fahn, A. 1979. Secretory Tissues in Plants. Academic Press, London, 302 pp.
Fajer, E.D., 1989. The effects of enriched CO2 atmospheres on plant-insect herbivore interactions: Growth responses of larvae of the specialist butterfly,Junonia coenia (Lepidoptera: Nymphalidae).Oecologia 81:514–520.
Fajer, E.D., Bowers, M.D., andBazzaz, F.A. 1989. The effects of enriched carbon dioxide atmospheres on plant-insect herbivore interactions.Science 243:1198–1200.
Fajer, E.D., Bowers, M.D., andBazzaz, F.A. 1992. The effect of nutrients and enriched CO2 environments on production of carbon-based allelochemicals inPlantago: A test of the carbon/nutrient balance hypothesis.Am. Nat. 140:707–723.
Feeny, P. 1976. Plant apparency and chemical defense, pp. 1–40,in J.W. Wallace and R.L. Mansell (eds.). Biochemical Interactions between Plants and Insects, Recent Advances in Phytochemistry, Vol. 10. Plenum Press, New York.
Fischer, N.H. 1986. The function of mono and sesquiterpenes as plant germination and growth regulators, pp. 203–218,in A.R. Putnam and C.-S. Tang (eds.). The Science of Allelopathy. John Wiley & Sons, New York.
Fischer, N.H. 1991. Plant terpenoids as allelopathic agents, pp. 377–398,in J.B. Harborne and F.A. Tomas-Barberan (eds.). Ecological Chemistry and Biochemistry of Plant Terpenoids, Annual Proceedings of the Phytochemical Society of Europe, Vol. 31. Clarendon Press, Oxford.
Flesch, V., Jacques, M., Cosson, L., Teng, B.P., Petiard, V., andBalz, J.P. 1992. Relative importance of growth and light level on terpene content ofGinkgo biloba.Phytochemistry 31:1941–1945.
Fox, L.R. 1981. Defense and dynamics in plant-herbivore systems.Am. Zool. 21:853–864.
Francis, M.J.O., andO'Connell, M. 1969. The incorporation of mevalonic acid into rose petal monoterpenes.Phytochemistry 8:1705–1708.
Funk, C., andCroteau, R. 1994. Diterpenoid resin acid biosynthesis in conifers: Characterization of two cytochrome P450-dependent monooxygenases and an aldehyde dehydrogenase involved in abietic acid biosynthesis.Arch. Biochem. Biophys. 308:258–266.
Funk, C., Koepp, A.E., andCroteau, R. 1992. Catabolism of camphor in tissue cultures and leaf disks of common sage (Salvia officinalis).Arch. Biochem. Biophys. 294:306–313.
Gambliel, H., andCroteau, R. 1984. Pinene cyclases I and II: Two enzymes from sage (Salvia officinalis) which catalyze stereospecific cyclizations of geranyl pyrophosphate to monoterpene olefins of opposite configuration.J. Biol. Chem. 259:740–748.
Gershenzon, J. 1984. Changes in the level of plant secondary metabolite production under water and nutrient stress, pp. 273–320,in B.N. Timmermann, C. Steelink, and F.A. Loewus (eds.). Phytochemical Adaptations to Stress, Recent Advances in Phytochemistry, Vol. 18. Plenum Press, New York.
Gershenzon, J. 1994. The cost of plant chemical defense against herbivory: A biochemical perspective, pp. 105–173,in E.A. Bernays (ed.). Insect-Plant Interactions, Vol. V. CRC Press, Boca Raton, Florida.
Gershenzon, J. andCroteau, R. 1990. Regulation of monoterpene biosynthesis in higher plants, pp. 99–160,in G.H.N. Towers and H.A. Stafford (eds.). Biochemistry of the Mevalonic Acid Pathway to Terpenoids, Recent Advances in Phytochemistry, Vol. 24. Plenum Press, New York.
Gershenzon, J., andCroteau, R. 1991. Terpenoids, pp. 165–219,in G.A. Rosenthal and M.R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites, Vol. 1, 2nd ed. Academic Press, San Diego.
Gershenzon, J., andCroteau, R. 1993. Terpenoid biosynthesis: The basic pathway and formation of monoterpenes, sesquiterpenes, and diterpenes, pp. 333–388,in T.S. Moore, Jr. (ed.). Lipid Metabolism In Plants. CRC Press, Boca Raton, Florida.
Gershenzon, J., Maffei, M., andCroteau, R. 1989. Biochemical and histochemical localization of monoterpene biosynthesis in the glandular trichomes of spearmint (Mentha spicata).Plant Physiol. 89:1351–1357.
Gershenzon, J., Murtagh, G.J., andCroteau, R. 1993. Absence of rapid terpene turnover in several diverse species of terpene-accumulating plants.Oecologia 96:583–592.
Gijzen, M., Lewinsohn, E., andCroteau, R. 1992. Antigenic cross-reactivity among monoterpene cyclases from grand fir and induction of these enzymes upon stem wounding.Arch. Biochem. Biophys. 294:670–674.
Gleizes, M., Pauly, G., Carde, J.-P., Marpeau, A., andBernard-Dagan, C. 1983. Monoterpene hydrocarbon biosynthesis by isolated leucoplasts ofCitrofortunella mitis.Planta 159:373–381.
Goodwin, T.W., andMercer, E.I. 1983. Introduction to Plant Biochemistry, 2nd ed. Pergamon Press, Oxford, 677 pp.
Graebe, J.E. 1987. Gibberellin biosynthesis and control.Annu. Rev. Plant Physiol. 38:419–465.
Gray, J.C. 1987. Control of isoprenoid biosynthesis in higher plants.Adv. Bot. Res. 14:25–91.
Grebenok, R.J., andAdler, J.H. 1991. Ecdysteroid distribution during development of spinach.Phytochemistry 30:2905–2910.
Groeneveld, H.W., Elings, J.C., andKoops, A.J. 1987. Mobilisation of reserves and the earliest synthesis of sterols and latex triterpenes during germination and early seedling growth ofEuphorbia lathyris.Physiol. Plant. 71:296–301.
Gulmon, S.L., andMooney, H.A. 1986. Costs of defense and their effects on plant productivity, pp. 681–698,in T.J. Givnish (ed.). On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, U.K.
Hallahan, T.W., andCroteau, R. 1988. Monoterpene biosynthesis: Demonstration of a geranyl pyrophosphate: sabinene hydrate cyclase in soluble enzyme preparations from sweet marjoram (Majorana hortensis).Arch. Biochem. Biophys. 264:618–631.
Hanover, J.W. 1966. Genetics of terpenes. I. Gene control of monoterpene levels inPinus monticola Dougl.Heredity 21:73–84.
Hasegawa, S., Herman, Z., Orme, E., andOu, P. 1986. Biosynthesis of limonoids inCitrus: Site and translocation.Phytochemistry 25:2783–2785.
Hawkins, A.J.S. 1991. Protein turnover: A functional appraisal.Funct. Ecol. 5:222–233.
Hefendehl, F.W., Underhill, E.W., andVon Rudloff, E. 1967. The biosynthesis of the oxygenated monoterpenes in mint.Phytochemistry 6:823–835.
Heldt, H.W., andFlugge, U.I. 1987. Subcellular transport of metabolites in plant cells, pp. 49–85,in D.D. Davies (ed.). The Biochemistry of Plants, A Comprehensive Treatise, Vol. 12, Physiology of Metabolism. Academic Press, San Diego.
Henry, M., Rochd, M., andBennini, B. 1991. Biosynthesis and accumulation of saponins inGypsophila paniculata.Phytochemistry 30:1819–1821.
Herms, D.A., andMattson, W.J. 1992. The dilemma of plants: To grow or defend.Q. Rev. Biol. 67:283–335.
Hopfinger, J.A., Kumamoto, J., andScora, R.W. 1979. Diurnal variation in the essential oils of Valencia orange leaves.Am. J. Bot. 66:111–115.
Janson, R.W. 1993. Monoterpene emissions from Scots pine and Norwegian spruce.J. Geophys. Res. 98:2839–2850.
Janzen, D.H. 1975. Behavior ofHymenaea courbaril when its predispersal seed predator is absent.Science 189:145–147.
Jensen, S.R. 1991. Plant iridoids, their biosynthesis and distribution in angiosperms, pp. 133–158,in J.B. Harborne and F.A. Tomas-Barberan (eds.). Ecological Chemistry and Biochemistry of Plant Terpenoids, Annual Proceedings of the Phytochemical Society of Europe, Vol. 31. Clarendon Press, Oxford.
Johnson, R.H., andLincoln, D.E. 1990. Sagebrush and grasshopper responses to atmospheric carbon dioxide concentration.Oecologia 84:103–110.
Johnson, R.H., andLincoln, D.E. 1991. Sagebrush carbon allocation patterns and grasshopper nutrition: The influence of CO2 enrichment and soil mineral limitation.Oecologia 87:127–134.
Kalinowska, M., andWojciechowski, Z.A. 1988. Substrate specificity of partially purified UDP-glucose: nuatigenin glucosyltransferase from oat leaves.Plant Sci. 55:239–245.
Karp, F., Harris, J.L., andCroteau, R. 1987. Metabolism of monoterpenes: Demonstration of the hydroxylation of (+)-sabinene to (+)-cis-sabinol by an enzyme preparation from sage (Salvia officinalis) leaves.Arch. Biochem. Biophys. 256:179–193.
Karp, F., Mihaliak, C.A., Harris, J.L., andCroteau, R. 1990. Monoterpene biosynthesis: Specificity of the hydroxylation of (−)-limonene by enzyme preparations from peppermint (Mentha piperita), spearmint (Mentha spicata) and perilla (Perilla frutescens) leaves.Arch. Biochem. Biophys. 276:219–226.
Keene, C.K., andWagner, G.J. 1985. Direct demonstration of duvatrienediol biosynthesis in glandular heads of tobacco trichomes.Plant Physiol. 79:1026–1032.
Kelsey, R.G., andVance, N.C. 1992. Taxol and cephalomannine concentrations in the foliage and bark of shade-grown and sun-exposedTaxus brevifolia trees.J. Nat. Prod. 55:912–917.
Kelsey, R.G., Reynolds, G.W., andRodriguez, E. 1984. The chemistry of biologically active constituents secreted and stored in plant glandular trichomes, pp. 187–241,in E. Rodriguez, P.L. Healey, and I. Mehta (eds.). Biology and Chemistry of Plant Trichomes. Plenum Press, New York.
Kjonaas, R., andCroteau, R. 1983. Demonstration that limonene is the first cyclic intermediate in the biosynthesis of oxygenatedp-menthane monoterpenes inMentha piperita and otherMentha species.Arch. Biochem. Biophys. 220:79–89.
Kjonaas, R., Martinkus-Taylor, C., andCroteau, R. 1982. Metabolism of monoterpenes: Conversion ofl-menthone tol-menthol andd-neomenthol by stereospecific dehydrogenases from pepperment (Mentha piperita) leaves.Plant Physiol. 69:1013–1017.
Kjonaas, R.B., Venkatachalam, K.V., andCroteau, R. 1985. Metabolism of monoterpenes: Oxidation of isopiperitenol to isopiperitenone, and subsequent isomerization to piperitenone by soluble enzyme preparations from peppermint (Mentha piperita) leaves.Arch. Biochem. Biophys. 238:49–60.
Kleinig, H. 1989. The role of plastids in isoprenoid biosynthesis.Annu. Rev. Plant Physiol. Plant Mol. Biol. 40:39–59.
Koops, A.J., andGroeneveld, H.W. 1991. Triterpenoid biosynthesis in the etiolated seedling ofEuphorbia lathyris L. Developmental changes and the regulation of local triterpenoid production.J. Plant Physiol. 138:142–149.
Krischik, V.A., andDenno, R.F. 1983. Individual, population, and geographic patterns in plant defense, pp. 463–512, in R.F. Denno and M.S. McClure (eds.). Variable Plants and Herbivores in Natural and Managed Systems. Academic Press, New York.
Lamb, B., Guenther, A., Gay, D., andWestberg, H. 1987. A national inventory of biogenic hydrocarbon emissions.Atmos. Environ. 21:1695–1705.
Lambers, H., andRychter, A.M. 1989. The biochemical background of variation in respiration rate: Respiratory pathways and chemical composition, pp. 199–225,in H. Lambers (ed.). Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants. SPB Academic Publishers, The Hague.
Langenheim, J.H., Stubblebine, W.H., Lincoln, D.E., andFoster, C.E. 1978. Implications of variation in resin composition among organs, tissues and populations in the tropical legumeHymenaea.Biochem. Syst. Ecol. 6:299–313.
Langenheim, J.H., Lincoln, D.E., Stubblebine, W.H., andGabrielli, A.C. 1982. Evolutionary implications of leaf resin pocket patterns in the tropical treeHymenaea (Caesalpinioideae: Leguminosae).Am. J. Bot. 69:595–607.
Lapinjoki, S.P., Elo, H.A., andTaipale, H.T. 1991. Development and structure of resin glands on tissues ofBetula pendula Roth. during growth.New Phytol. 117:219–223.
Lawrence, B.M. 1992. Chemical components of Labiatae oils and their exploitation, pp. 399–436,in R.M. Harley and T. Reynolds (eds.). Advances in Labiate Science. Royal Botanic Gardens, Kew, U.K.
Lerdau, M. 1993. Formal equivalence among resource allocation models: What is the appropriate currency?Funct. Ecol. 7:507–508.
Lerdau, M. Litvak, M., andMonson, R. 1994. Plant chemical defense: Monoterpenes and the growth-differentiation balance hypothesis.Trends Ecol. Evol. 9:58–61.
Lewinsohn, E., Gijzen, M., andCroteau, R. 1992. Wound-inducible pinene cyclase from grand fir: Purification, characterization and renaturation after SDS-PAGE.Arch. Biochem. Biophys. 293:167–173.
Lincoln, D.E., andCouvet, D. 1989. The effect of carbon supply on allocation to allelochemicals and caterpillar consumption of peppermint.Oecologia 78:112–114.
Loomis, W.E. 1932. Growth-differentiation balance vs. carbohydrate-nitrogen ratio.Proc. Am. Soc. Hortic. Sci. 29:240–245.
Lorio, P.L., Jr. 1986. Growth-differentiation balance: A basis for understanding southern pine beetle-tree interactions.For. Ecol. Manage. 14:259–273.
Lorio, P.L., Jr. 1988. Growth differentiation-balance relationships in pines affect their resistance to bark beetles (Coleoptera: Scolytidae), pp. 73–92,in W.J. Mattson, J. Levieux, and C. Bernard-Dagan (eds.). Mechanisms of Woody Plant Defenses Against Insects, Search for Pattern. Springer-Verlag, New York.
Loveys, B.R., Robinson, S.P., Brophy, J.J., andChacko, E.K. 1992. Mango sapburn: Components of fruit sap and their role in causing skin damage.Aust. J. Plant Physiol. 19:449–457.
Mahlberg, P.G., Davis, D.G., Galitz, D.S., andManners, G.D. 1987. Laticifers and the classification ofEuphorbia: The chemotaxonomy ofEuphorbia esula L.Bot. J. Linn. Soc. 94:165–180.
Mariani, P., Cappelletti, E.M., Campoccia, D., andBaldan, B. 1989. Oil cell ultrastructure and development inLiriodendron tulipifera L.Bot. Gaz. 150:391–396.
Marner, F.-J., andKerp, B. 1992. Composition of iridals, unusual triterpenoids from sword-lilies, and the seasonal dependence of their content in various parts of differentIris species.Z. Naturforsch. 47c:21–25.
Mathur, A.K., Ahuja, P.S., Pandey, B., Kukreja, A.K., andMandal, S. 1988. Screening and evaluation of somaclonal variations for quantitative and qualitative traits in an aromatic grass,Cymbopogon winterianus Jowitt.Plant Breeding 101:321–334.
McCullough, D.G., andKulman, H.M. 1991. Differences in foliage quality of young jack pine (Pinus banksiana Lamb.) on burned and clearcut sites: Effects on jack pine budworm (Choristoneura pinus pinus Freeman).Oecologia 87:135–145.
McDermitt, D.K., andLoomis, R.S. 1981. Elemental composition of biomass and its relation to energy content, growth efficiency, and growth yield.Ann. Bot. 48:275–290.
McKey, D. 1979. The distribution of secondary compounds within plants, pp. 55–133,in G.A. Rosenthal and D.H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites, 1st ed. Academic Press, New York.
Merino, J., Field, C., andMooney, H.A. 1984. Construction and maintenance costs of Mediterranean-climate evergreen and deciduous leaves. II. Biochemical pathway analysis.Oecol. Plant. 5:211–229.
Metivier, J., andViana, A.M. 1979. The effect of long and short day length upon the growth of whole plants and the level of soluble proteins, sugars, and stevioside in leaves ofStevia rebaudiana Bert.J. Exp. Bot. 30:1211–1222.
Meyberg, M., Krohn, W., Brümmer, B., andKristen, U. 1991. Ultrastructure and secretion of glandular trichomes of tobacco leaves.Flora 185:357–363.
Mihaliak, C.A., andLincoln, D.E. 1989. Changes in leaf mono-and sesquiterpene metabolism with nitrate availability and leaf age inHeterotheca subaxillaris.J. Chem. Ecol. 15:1579–1588.
Mihaliak, C.A., Gershenzon, J., andCroteau, R. 1991. Lack of rapid monoterpene turnover in rooted plants: Implications for theories of plant chemical defense.Oecologia 87:373–376.
Monfar, M., Caelles, C., Balcells, L., Ferrer, A., Hegardt, F.G., andBoronat, A. 1990. Molecular cloning and characterization of plant 3-hydroxy-3-methylglutaryl coenzyme A reductase, pp. 83–97,in G.H.N. Towers and H.A. Stafford (eds.). Biochemistry of the Mevalonic Acid Pathway to Terpenoids, Recent Advances in Phytochemistry, Vol. 24. Plenum Press, New York.
Morrow, P.A., andFox, L.R. 1980. Effects of variation inEucalyptus essential oil yield on insect growth and grazing damage.Oecologia 45:209–219.
Muller, W.H. 1986. Allelochemical mechanisms in the inhibition of herbs by chaparral shrubs, pp. 189–199,in A.R. Putnam and C.-S. Tang (eds.). The Science of Allelopathy. John Wiley & Sons, New York.
Munck, S.L., andCroteau, R. 1990. Purification and characterization of the sesquiterpene cyclase patchoulol synthase fromPogostemon cablin.Arch. Biochem. Biophys. 282:58–64.
Muzika, R.M., Pregitzer, K.S., andHanover, J.W. 1989. Changes in terpene production following nitrogen fertilization of grand fir [Abies grandis (Dougl.) Lindl.] seedlings.Oecologia 80:485–489.
Njar, V.C.O., Arnold, L.M., Banthorpe, D.V., Branch, S.A., Christie, A.C., andMarsh, D.C. 1989. Metabolism of exogenous monoterpenes and their epoxides in seedlings ofPinus pinaster Ait.J. Plant Physiol. 135:628–630.
Ohigashi, H., Wagner, M.R., Matsumura, F., andBenjamin, D.M. 1981. Chemical basis of differential feeding behavior of the larch sawfly,Pristiphora erichsonii (Hartig).J. Chem. Ecol. 7:599–614.
Paczkowski, C., andWojciechowski, Z.A. 1988. The occurrence of UDPG-dependent glucosyltransferase specific for sarsasapogenin inAsparagus officinalis.Phytochemistry 27:2743–2747.
Paterson-Jones, J.C., Gilliland, M.G., andVan Staden, J. 1990. The biosynthesis of natural rubber.J. Plant Physiol. 136:257–263.
Penning de Vries, F.W.T., Brunsting, A.H.M., andVan Laar, H.H. 1974. Products, requirements and efficiency of biosynthesis: A quantitative approach.J. Theor. Biol. 45:339–377.
Platt, K.A., andThomson, W.W. 1992. Idioblast oil cells of avocado: Distribution, isolation, ultrastructure, histochemistry, and biochemistry.Int. J. Plant Sci. 153:301–310.
Polonsky, J., Bhatnagar, S.C., Griffiths, D.C., Pickett, J.A., andWoodcock, C.M. 1989. Activity of quassinoids as antifeedants against aphids.J. Chem. Ecol. 15:993–998.
Porter, J.W., andSpurgeon, S.L. (eds.) 1981. Biosynthesis of Isoprenoid Compounds, Vols. 1 and 2. John Wiley & Sons, New York, 558 pp. and 552 pp.
Puritch, G.S., andNijholt, W.W. 1974. Occurrence of juvabione-related compounds in grand fir and Pacific silver fir infested by balsam woolly aphid.Can. J. Bot. 52:585–587.
Putievsky, E., Ravid, U., Snir, N., andSanderovich, D. 1984. The essential oils from cultivated bay laurel.Isr. J. Bot. 33:47–52.
Quispel, A., Svendsen, A.B., Schripsema, J., Baas, W.J., Erkelens, C., andLugtenburg, J. 1989. Identification of dipterocarpol as isolation factor for the induction of primary isolation ofFrankia from root nodules ofAlnus glutinosa (L.) Gaertner.Mol. Plant-Microbe Inter. 2:107–112.
Rajaonarivony, J.I.M., Gershenzon, J., andCroteau, R. 1992. Characterization and mechanism of (4S)-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha × piperita).Arch. Biochem. Biophys. 296:49–57.
Rausher, M.D. 1992. Natural selection and the evolution of plant-insect interactions, pp. 20–80,in B.D. Roitberg and M.B. Isman (eds.). Insect Chemical Ecology, An Evolutionary Approach. Chapman and Hall, New York.
Regnier, F.E., Waller, G.R., Eisenbraun, E.J., andAuda, H. 1968. The biosynthesis of methylcyclopentane monoterpenoids. II. Nepetalactone.Phytochemistry 7:221–230.
Reichardt, P.B., Bryant, J.P., Clausen, T.P., andWieland, G.D. 1984. Defense of winterdormant Alaska paper birch against snowshoe hares.Oecologia 65:58–69.
Reichardt, P.B., Chapin, F.S., III, Bryant, J.P., Mattes, B.R., andClausen, T.P. 1991. Carbon/nutrient balance as a predictor of plant defense in Alaskan balsam poplar: Potential importance of metabolite turnover.Oecologia 88:401–406.
Reynolds, A.G., andWardle, D.A. 1989. Influence of fruit microclimate on monoterpene levels of Gewürztraminer.Am. J. Enol. Vitic. 40:149–154.
Rhoades, D.F. 1979. Evolution of plant chemical defense against herbivores, pp. 3–54,in G.A. Rosenthal and D.H. Janzen (eds.). Herbivores: their Interaction with Secondary Plant Metabolites, 1st ed. Academic Press, New York.
Rogers, S., Wells, R., andRechsteiner, M. 1986. Amino acid sequences common to rapidly degraded proteins: The PEST hypothesis.Science 234:364–368.
Ross, J.D., andSombrero, C. 1991. Environmental control of essential oil production in Mediterranean plants, pp. 83–94,in J.B. Harborne and F.A. Tomas-Barberan (eds.). Ecological Chemistry and Biochemistry of Plant Terpenoids, Annual Proceedings of the Phytochemical Society of Europe, Vol. 31. Clarendon Press, Oxford.
Rousi, M., Tahvanainen, J., Henttonen, H., andUotila, I. 1993. Effect of shading and fertilization on resistance of winter-dormant birch (Betula pendula) to voles and hares.Ecology 74:30–38.
Roy, A.T., andDe, D.N. 1992. Studies on differentiation of laticifers through light and electron microscopy inCalotropis gigantea (Linn.) R.Br.Ann. Bot. 70:443–449.
Russin, W.A., Uchytil, T.F., Feistner, G., andDurbin, R.D. 1988. Developmental changes in content of foliar secretory cavities ofTagetes erecta (Asteraceae).Am. J. Bot. 75:1787–1793.
Russin, W.A., Uchytil, T.F., andDurbin, R.D. 1992. Isolation of structurally intact secretory cavities from leaves of African marigold,Tagetes erecta L. (Asteraceae).Plant. Sci. 85:115–119.
Schindler, T., andKotzias, D. 1989. Comparison of monoterpene volatilization and leaf-oil composition of conifers.Naturwissenschaften 76:475–476.
Schnepf, E. 1974. Gland cells, pp. 331–357,in A.W. Robards (ed.). Dynamic Aspects of Plant Ultrastructure. McGraw-Hill, New York.
Schulze-Siebert, D. andSchultz, G. 1987. Full autonomy in isoprenoid synthesis in spinach chloroplasts.Plant Physiol. Biochem. 25:145–153.
Scora, R.W., andMann, J.D. 1967. Essential oil synthesis inMonarda punctata.Lloydia 30:236–241.
Seaman, F., Bohlmann, F., Zdero, C., andMabry, T.J. 1990. Diterpenes of Flowering Plants, Compositae (Asteraceae). Springer-Verlag, New York, 638 pp.
Seigler, D., andPrince, P.W. 1976. Secondary compounds in plants: Primary functions.Am. Nat. 110:101–105.
Shomer, I., andErner, Y. 1989. The nature of oleocellosis in citrus fruits.Bot. Gaz. 150:281–288.
Simms, E.L. 1992. Costs of plant resistance to herbivory, pp. 392–425,in R.S. Fritz and E.L. Simms (eds.). Plant Resistance to Herbivores and Pathogens: Ecology, Evolution, and Genetics. University of Chicago Press, Chicago.
Simms, E.L., andFritz, R.S. 1990. The ecology and evolution of host-plant resistance to insects.Trends Ecol. Evol. 5:356–360.
Simms, E.L., andRausher, M.D. 1987. Costs and benefits of plant resistance to herbivory.Am. Nat. 130:570–581.
Singh, N., andLuthra, R. 1987. Sucrose metabolism and essential oil accumulation during lemongrass (Cymbopogon flexuosus Stapf.) leaf development.Plant Sci. 57:127–133.
Singh, N., Luthra, R., andSangwan, R.S. 1990. Oxidative pathways and essential oil biosynthesis in the developingCymbopogon flexuosus leaf.Plant Physiol. Biochem. 28:703–710.
Singh, N., Luthra, R., andSangwan, R.S. 1991. Mobilization of starch and essential oil biogenesis during leaf ontogeny of lemongrass (Cymbopogon flexuosus Stapf.).Plant Cell Physiol. 32:803–811.
Singh, V.P., Chatterjee, B.N., andSingh, D.V. 1989. Response of mint species to nitrogen fertilization.J. Agric. Sci. 113:267–271.
Skogsmyr, I., andFagerstrom, T. 1992. The cost of anti-herbivory defence: An evaluation of some ecological and physiological factors.Oikos 64:451–457.
Smyrl, T.G., andLemaguer, M. 1980. Solubilities of terpenic essential oil components in aqueous solutions.J. Chem. Eng. Data 25:150–152.
Spencer, A., Hamill, J.D., andRhodes, M.J.C. 1993. In vitro biosynthesis of monoterpenes byAgrobacterium transformed shoot cultures of twoMentha species.Phytochemistry 32:911–919.
Stahl-Biskup, E., andWichtmann, E.-M. 1991. Composition of the essential oils from roots of some Apiaceae in relation to the development of their oil duct systems.Flavour Fragr. J. 6:249–255.
Sukhov, G.V. 1958. The use of radiocarbon in the study of biosynthesis of terpenes, pp. 535–547,in R.C. Extermann (ed.). Radioisotopes in Scientific Research. Pergamon Press, New York.
Swiezewska, E., Dallner, G., Andersson, B., andErnster, L. 1993. Biosynthesis of ubiquinone and plastoquinone in the endoplasmic reticulum-Golgi membranes of spinach leaves.J. Biol. Chem. 268:1494–1499.
Tabata, M., Tanaka, S., Cho, H.J., Uno, C., Shimakura, J., Ito, M., Kamisako, W., andHonda, C. 1993. Production of anti-allergic triterpene, bryonolic acid, by plant cell cultures.J. Nat. Prod. 56:165–174.
Takabayashi, J., Dicke, M., andPosthumus, M.A. 1991. Induction of indirect defence against spider-mites in uninfested lima bean leaves.Phytochemistry 30:1459–1462.
Tallamy, D.W., andRaupp, M.J. (eds.). 1991. Phytochemical Induction by Herbivores. John Wiley & Sons, New York, 431 pp.
Tanaka, S., Yamaura, T., Shigemoto, R., andTabata, M. 1989. Phytochrome-mediated production of monoterpenes in thyme seedlings.Phytochemistry 28:2955–2957.
Thomson, W.W., Platt-Aloia, K.A., andEndress, A.G. 1976. Ultrastructure of oil gland development in the leaf ofCitrus sinensis L.Bot. Gaz. 137:330–340.
Threlfall, D.R., andWhitehead, I.M. 1991. Terpenoid phytoalexins: Aspects of biosynthesis, catabolism, and regulation, pp. 159–208,in J.B. Harborne and F.A. Tomas-Barberan (eds.). Ecological Chemistry and Biochemistry of Plant Terpenoids, Annual Proceedings of the Phytochemical Society of Europe, Vol. 31. Clarendon Press, Oxford.
Tingey, D.T., Turner, D.P., andWeber, J.A. 1991. Factors controlling the emissions of monoterpenes and other volatile organic compounds, pp. 93–119,in T.D. Sharkey, E.A. Holland, and H.A. Mooney (eds.). Trace Gas Emissions by Plants. Academic Press, San Diego.
Tisdale, R.A., andNebeker, T.E. 1992. Resin flow as a function of height along the bole of loblolly pine.Can. J. Bot. 70:2509–2511.
Towers, G.H.N., andStafford, H.A. (eds.). 1990. Biochemistry of the Mevalonate Pathway to Terpenoids, Recent Advances in Phytochemistry, Vol. 24. Plenum Press, New York, 341 pp.
Tuomi, J., Niemela, P., Chapin, F.S., III, Bryant, J.P., andSiren, S. 1988. Defensive responses of trees in relation to their carbon/nutrient balance, pp. 57–72,in W.J. Mattson, J. Levieux, and C. Bernard-Dagan (eds.). Mechanisms of Woody Plant Defenses Against Insects, Search for Pattern. Springer-Verlag, New York.
Tyson, B.J., Dement, W.A., andMooney, H.A. 1974. Volatilisation of terpenes fromSalvia mellifera.Nature 252:119–120.
Van Wassenhove, F.A., Dirinck, P.J., Schamp, N.M., andVulsteke, G.A. 1990. Effect of nitrogen fertilizers on celery volatiles.J. Agric. Food Chem. 38:220–226.
Vierstra, R.D. 1993. Protein degradation in plants.Annu. Rev. Plant Physiol. Plant Mol. Biol. 44:385–410.
Vogeli, U., andChappell, J. 1990. Regulation of a sesquiterpene cyclase in cellulase-treated tobacco cell suspension cultures.Plant Physiol. 94:1860–1866.
Wagschal, K., Savage, T.J., andCroteau, R. 1991. Isotopically sensitive branching as a tool for evaluating multiple product formation by monoterpene cyclases.Tetrahedron 47:5933–5944.
Waterman, P.G., andMole, S. 1989. Extrinsic factors influencing production of secondary metabolites in plants, pp. 107–134,in E.A. Bernays (ed.). Insect-Plant Interactions, Vol. 1, CRC Press, Boca Raton, Florida.
Weidenhamer, J.D., Macias, F.A., Fischer, N.H., andWilliamson, G.B. 1993. Just how insoluble are monoterpenes?J. Chem. Ecol. 19:1799–1807.
Werker, E., andFahn, A. 1981. Secretory hairs ofInula viscosa (L.) Ait.—development, ultrastructure, and secretion.Bot. Gaz. 142:461–476.
Werker, E., Ravid, U., andPutievsky, E. 1985. Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae.Isr. J. Bot. 34:31–45.
Werker, E., Putievsky, E., Ravid, U., Dudai, N., andKatzir, I. 1993. Glandular hairs and essential oil in developing leaves ofOcimum basilicum L. (Lamiaceae).Ann. Bot. 71:43–50.
West, C.A. 1981. Biosynthesis of diterpenes, pp. 375–411,in J.W. Porter and S.L. Spurgeon (eds.). Biosynthesis of Isoprenoid Compounds, Vol. 1. John Wiley & Sons, New York.
Williams, K., Percival, F., Merino, J., andMooney, H.A. 1987. Estimation of tissue construction cost from heat of combustion and organic nitrogen content.Plant Cell Environ. 10:725–734.
Yamaura, T., Tanaka, S., andTabata, M. 1989. Light-dependent formation of glandular trichomes and monoterpenes in thyme seedlings.Phytochemistry 28:741–744.
Yates, J.L., andPeckol, P. 1993. Effects of nutrient availability and herbivory on polyphenolics in the seaweedFucus vesiculosus.Ecology 74:1757–1766.
Zambou, K., Spyropoulos, C.G., Chinou, I., andKontos, F. 1993. Saponin-like substances inhibit α-galactosidase production in the endosperm of fenugreek seeds, a possible regulatory role in endosperm galactomannan degradation.Planta 189:207–212.
Zangerl, A.R., andBazzaz, F.A. 1992. Theory and pattern in plant defense allocation, pp. 363–391,in R.S. Fritz and E.L. Simms (eds.). Plant Resistance to Herbivores and Pathogens: Ecology, Evolution, and Genetics. University of Chicago Press, Chicago.
Zimowski, J. 1991. Occurrence of a glucosyltransferase specific for solanidine in potato plants.Phytochemistry 30:1827–1831.
Zimowski, J. 1992. Specificity and some other properties of cytosolic and membranous UDPGlc: 3ß-hydroxysteroid glucosyltransferases fromSolanum tuberosum leaves.Phytochemistry 31:2977–2981.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gershenzon, J. Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol 20, 1281–1328 (1994). https://doi.org/10.1007/BF02059810
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02059810