Abstract
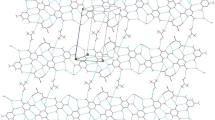
The crystal structures of four dimethyl sulphoxide (DMSO) inclusion compounds with different carboxylic acid hosts,1–4, have been studied by single crystal X-ray analysis. Crystals of thetrans-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboxylic acid inclusion compound (1a), [1 · DMSO (1: 1)] show monoclinic (P21/n) symmetry with the unit cell dimensionsa = 11.522(4),b = 18.658(2),c = 8.709(1) Å and β = 98.92(2)°. The clathrate of the 9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboxylic acid (2a), [2 · DMSO (1: 2)] is triclinic (PĪ) with the cell dimensionsa = 15.043(7),b =9.657(4),c = 8.118(7) Å, α = 101.81(5), β = 96.05(4) and γ = 100.04(4)°. Triclinic (PĪ) symmetry is shown also by the inclusion compound of 9,10-dihydro-9,10-ethanoanthracene-11-monocarboxylic acid (3a) [3 · DMSO (1:1)] with the cell dimensionsa=6.3132(1),b=7.9846(2),c=17.5314(4) Å, α = 96.46(2), β = 87.08(2) and γ = 106.02(2)°. The 9,9′-bianthryl-2-monocarboxylic acid clathrate (4a) [4 · DMSO (1:1)] is monoclinic (P21/n) and the cell dimensions area = 19.625(18),b = 8.817(1),c = 14.076(8) Å and β = 97.92(6)°. In all these structures, the hosts show the same basic recognition pattern for the DMSO guest, involving a strong O-H ... O bond from the COON to the S=O group, and a possible C-H ... O type interaction between the carbonyl O atom of the host and a CH3 group of the guest. The crystals consist of discrete host-guest aggregates which are mainly held together by weak intermolecular interactions of the Van der Waals' type. The stoichiometries of the aggregates are, however, different.
Similar content being viewed by others
References
E. Weber (Ed.):Molecular Inclusion and Molecular Recognition — Clathrates I (Topics in Current Chemistry, vol. 140). Springer-Verlag, Berlin-Heidelberg-New York (1987); part II (vol. 149) (1988).
R. M. Izatt and J. J. Christensen (Eds.):Synthesis of Macrocycles - The Design of Selective Complexing Agents (Progress in Macrocyclic Chemistry, vol. 3), Wiley, New York (1987).
G. Van Binst (Ed.):Design and Synthesis of Organic Molecules Based on Molecular Recognition. Springer Verlag, New York (1986).
J. L. Atwood, J. E. D. Davies and D. D. MacNicol (Eds.):Inclusion Compounds vols. I–III. Academic Press, London (1984).
J. M. Lehn:Angew. Chem. 100, 91 (1988);Angew. Chem., Int. Ed. Engl. 27, 89 (1988).
D. J. Cram:Angew. Chem. 100, 1041 (1988);Angew. Chem., Int. Ed. Engl. 27, 1009 (1988).
H. Grünewald (Ed.):Chemistry for the Future. Pergamon Press, Oxford-New York (1984).
E. Weber and H. P. Josel:J. Incl. Phenom. 1, 79 (1983).
E. Weber, I. Csöregh, B. Stensland, and M. Czugler:J. Am. Chem. Soc. 106, 3297 (1984).
M. Czugler, J. J. Stezowski, and E. Weber:J. Chem. Soc., Chem. Commun. 154 (1983).
I. Csöregh, A. Sjögren, M. Czugler, and E. Weber:J. Chem. Soc., Perkin Trans. 2, 507 (1986).
E. Weber, M. Hecker, E. Koepp, W. Orlia, M. Czugler, and I. Csöregh:J. Chem. Soc., Perkin Trans. 2 (1988), 1251.
E. Weber, I. Csöregh, J. Ahrendt, S. Finge, and M. Czugler:J. Org. Chem. 53, 5831 (1988).
F. Bell and D. H. Waring:J. Chem. Soc. 2689 (1949).
P. Main, S. J. Fiske, S. E. Hull, L. Lessinger, G. Germain, J.-P. Declerq, and M. M. Woolfson:MULTAN 80. A System of Computer Programs for the Automatic Solution of Crystal Structures from X-Ray Diffraction Data. University of York, England (1980).
G. M. Sheldrick:SHELXS 84. Program for Crystal Structure Solution. University of Göttingen, FR-Germany, personal communication.
G. M. Sheldrick:SHELX 76. Program for Crystal Structure Determination. Univ. of Cambridge, England (1976).
M. Czugler, E. Weber, and J. Ahrendt:J. Chem. Soc., Chem. Commun. 1632 (1984).
I. Csöregh, M. Czugler, and E. Weber: inMolecular Structure: Chemical Reactivity and Biological Activity (Ed. J. J. Stezowski) pp. 390–395. Oxford University Press (1988).
O. Kennard: inInternational Tables for X-ray Crystallography, vol. III, pp. 275–276. Kynoch Press, Birmingham, England (1968) [Distributed by Kluwer Academic Publishers, Dordrecht and Boston.]
S. N. Vinogradov and R. H. Linnell (Eds.):Hydrogen Bonding, Van Nostrand Reinhold, New York (1971).
R. Taylor and O. Kennard:J. Am. Chem. Soc. 104, 5063 (1982).
J. R. Damewood, Jr., J. J. Urban, T. C. Williamson, and A. L. Rheingold:J. Org. Chem. 53, 167 (1988).
R. A. Kumpf and J. R. Damewood, Jr.:J. Chem. Soc., Chem. Commun. 621 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Csöregh, I., Czugler, M., Ertan, A. et al. Solid-state binding of dimethyl sulphoxide involving carboxylic host molecules. X-ray crystal structures of four inclusion species. J Incl Phenom Macrocycl Chem 8, 275–287 (1990). https://doi.org/10.1007/BF01041884
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01041884