Summary
-
1.
The auditory system of the tettigoniid,Conocephalus nigropleurum was examined using whole auditory nerve responses (averaged summed action potentials (SAPs)) and intracellularly recorded single cells (receptor and interneurone) to pure tone stimuli and con- and heterospecific (C. brevipennis) songs.
-
2.
The morphology of the auditory tracheal system and crista acustica is documented forC. nigropleurum. A broadly open thoracic spiracle (stigma) leads, via a horn, camera and foreleg trachea to a crista acustica of 28 receptor cells.
-
3.
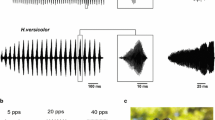
C. nigropleurum's auditory sensitivity is broadly tuned to 15–35 kHz which is below the peak frequency band of its calling song (30–36 kHz). The ear possesses a dynamic range of at least 30 dB and pulsed sounds with short (<0.5 ms) onset times or long interpulse periods (>200 ms) elicit maximal responses. We suggest this is caused by increased auditory receptor firing synchrony to these types of sounds and may facilitate the location of such sounds. There is, however, discrepancy between the pulse periods that elicit maximal SAP amplitudes and those found within the normal calling song, a phenomenon which may be related to the chorusing behaviour of this species.
-
4.
Polar directional plots indicate thatC. nigropleurum is most sensitive to sounds originating posterior to the opening of the prothoracic auditory stigma; this may be caused by the configuration of the prothoracic pronotum. We found no pronounced sensitivity at points facing the tympanal slits.
-
5.
Intracellular recordings reveal prothoracic receptors with widely decussate and narrowly branched termini. No receptor tested was able to track the intra-phonatome pulses (tooth strike sounds) of either species and we conclude that the phonatome is the finest resoluble component of the song.
-
6.
An acoustically-activated interneurone (101) was recorded that resembles both the gryllid int-2 (Omega) cell and that of another tettigoniid. We suggest that tonic interneuronal firing caused by the multiple singer chorused song of either species activates the initial stages of phonotaxis in receptiveC. nigropleurum females but individual species recognition may depend at least partially on the discrimination of phonatome rates.
Similar content being viewed by others
Abbreviations
- dB SPL :
-
decibel re 20 μPa
- MaPT :
-
major pulse train
- MiPT :
-
minor pulse train
- SAP :
-
summed action potential
- BF :
-
best frequency
References
Adam L-J (1977a) The oscillating summed action potential of an insect's auditory nerve (Locusta migratoria, Acrididae) I. Its original form and time constancy. Biol Cybern 26:241–247
Adam L-J (1977b) The oscillating summed action potential of an insect's auditory nerve (Locusta migratoria, Acrididae) II. Underlying spike pattern and causes of spike synchronization. Biol Cybern 28:109–111
Alexander RD (1961) Aggressiveness, territoriality and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 17:130–223
Ander K (1939) Vergleichend-anatomische und phylogenetische Studien über die Ensifera (Saltatoria). Opuscula Entomol Suppl II (Unpublished translation by TH Hubbell)
Bailey WJ, Morris GK (1986) Confusion of phonotaxis by masking sounds in the bushcricketConocephalus brevipennis (Tettigoniidae: Conocephalinae). Ethology 73:19–28
Bailey WJ, Stephen RO (1978) Directionality and auditory slit function. A theory of hearing in bushcrickets. Science 201:633–634
Bailey WJ, Stephen RO (1984) Auditory acuity in the orientation behaviour of the bushcricketPachysagella australis Walker (Orthoptera, Tettigoniidae, Saginae). Anim Behav 32:816–829
Belwood JJ, Morris GK (1987) Bat predation and its influence on calling behavior in neotropical katydids. Science 238:64–67
Boyan GS (1984) Neural mechanisms of auditory information processing by identified interneurones in Orthoptera. J Insect Physiol 30:27–41
Boyd P, Lewis B (1983) Peripheral auditory directionality in the cricket (Gryllus campestris L.,Teleogryllus oceanicus Le Guillou). J Comp Physiol 153:523–532
Casaday GB, Hoy RR (1977) Auditory interneurons in the cricketTeleogryllus oceanicus: physiological and anatomical properties. J Comp Physiol 121:1–13
Greenfield MD, Shaw KC (1983) Adaptive significance of chorusing with special reference to the Orthoptera. In: Gwynne DT, Morris GK (eds) Orthopteran mating systems: sexual competition in a diverse group of insects. Westview Press, Boulder, pp 1–27
Gwynne DT (1982) Mate selection by female katydids (Orthoptera: Tettigoniidae,Conocephalus nigropleurum). Anim Behav 30:734–738
Gwynne DT, Morris GK (1986) Heterospecific recognition and behavioral isolation in acoustic Orthoptera (Insecta). Evol Theory 8:33–38
Helversen D von, Helversen O von (1983) Species recognition and acoustic localization in acridid grasshoppers: a behavioral approach. In: Huber F, Markl H (eds) Neuroethology and behavioral physiology. Springer, Berlin Heidelberg New York, pp 95–107
Hill KG, Boyan GS (1976) Directional hearing in crickets. Nature 262:390–391
Hill KG, Boyan GS (1977) Sensitivity to frequency and direction of sound in the auditory system of crickets (Gryllidae). J Comp Physiol 121:79–97
Hill KG, Oldfield BP (1981) Auditory function in Tettigoniidae (Orthoptera: Ensifera). J Comp Physiol 142:169–180
Huber F (1983) Neural correlates of orthopteran and cicada phonotaxis. In: Huber F, Markl H (eds) Neuroethology and behavioral physiology. Springer, Berlin Heidelberg New York, pp 108–135
Klein MS (1955) Ionophone ou haut-parleur ionique. In: Busnel R-G (ed) Colloque sur l'acoustique des orthoptères. Institut national de la recherche agronomique, Paris, pp 46–49
Latimer W (1981) The acoustic behaviour ofPlatycleis albopunctata (Goeze) (Orthoptera: Tettigoniidae). Behaviour 76:182–206
Michelsen A (1971) The physiology of the locust ear. III. Acoustical properties of the intact ear. Z Vergl Physiol 71:102–128
Morris GK (1971) Aggression in male conocephaline grasshoppers (Tettigoniidae). Anim Behav 19:132–137
Morris GK, Fullard JH (1983) Random noise and congeneric discrimination inConocephalus nigropleurum (Orthoptera: Tettigoniidae). In: Gwynne DT, Morris GK (eds) Orthopteran mating systems: sexual competition in a diverse group of insects. Westview Press, Boulder, pp 73–96
Morris GK, Kerr GE, Fullard JH (1978) Phonotactic preference of female meadow katydids (Orthoptera, Tettigoniidae:Conocephalus nigropleurum). Can J Zool 56:1479–1487
Morris GK, Pipher RE (1967) Tegminal amplifiers and spectrum consistencies inConocephalus nigropleurum (Bruner), Tettigoniidae. J Insect Physiol 13:1075–1085
Nocke H (1975) Physical and physiological properties of the tettigoniid (‘grasshopper’) ear. J Comp Physiol 100:25–57
Oldfield BP (1983) Central projections of primary auditory fibres in Tettigoniidae (Orthoptera: Ensifera). J Comp Physiol 151:389–395
Oldfield BP (1985) The tuning of auditory receptors in bushcrickets. Hearing Res 17:27–35
Oldfield BP, Kleindienst HU, Huber F (1986) Physiology and tonotopic organization of auditory receptors in the cricketGryllus bimaculatus De Geer. J Comp Physiol A 159:457–464.
Otte D (1977) Communication in Orthoptera. In: Sebeok TA (ed) How animals communicate. Indiana Univ. Press, Bloomington, pp 334–361
Pipher RE, Morris GK (1974) Frequency modulation inConocephalus nigropleurum, the black-sided meadow katydid (Orthoptera: Tettigoniidae). Can Entomol 106:997–1001
Robertson RM, Pearson KG (1983) Interneurons in the flight system of the locust: distribution, connections and resetting properties. J Comp Neurol 215:33–50
Römer H (1985) Anatomical representation of frequency and intensity in the auditory system of Orthoptera. In: Kalmring K, Elsner N (eds) Acoustic and vibrational communication in insects. Paul Parey, Berlin, pp 25–32
Römer H, Marquart V (1984) Morphology and physiology of auditory interneurons in the metathoracic ganglion of the locust. J Comp Physiol A 155:249–262
Römer H, Rheinlaender J (1983) Electrical stimulation of the tympanal nerve as a tool for analysing the responses of auditory interneurons in the locust. J Comp Physiol 152:289–296
Ronacher B, Römer H (1985) Spike synchronization of tympanic receptor fibres in a grasshopper (Chorthippus biguttulus L., Acrididae). A possible mechanism for detection of short gaps in model songs. J Comp Physiol A 157:631–642
Sakaluk S, Belwood JJ (1984) Gecko phonotaxis to cricket calling song: a case of satellite predation. Anim Behav 32:659–662
Snodgrass RE (1935) Principles of insect morphology. McGraw-Hill, New York
Stephen RO, Bailey WJ (1982) The bioacoustics of the ear of the bushcricketHemisaga (Saginae). J Acoust Soc Am 72:13–25
Stewart WW (1978) Functional connections between cells as revealed by a highly fluorescent naphthalimide tracer. Cell 14:741–759
Suga N (1966) Ultrasonic production and its reception in some neotropical Tettigoniidae. J Insect Physiol 12:1039–1050
Walker TW, Dew D (1972) Wing movements of calling katydids: fiddling finese. Science 178:174–176
Wohlers DW, Huber F (1978) Intracellular recording and staining of cricket auditory interneurons (Gryllus campestris L.,Gryllus bimaculatus DeGeer). J Comp Physiol 127:11–28
Zar JH (1984) Biostatistical analyses, 2nd edn. Prentice-Hall, Englewood Cliffs
Zeuner FE (1936) The prothoracic tracheal apparatus of Saltatoria (Orthoptera). Proc R Entomol Soc Lond (A) 11:11–21
Zhantiev RD, Kalinkina IN, Chukanov VS (1975) Characteristics of the directional sensitivity of the tympanal organs in the cricketGryllus bimaculatus Deg. (Orthoptera, Gryllidae). Entomol Obozr 54:249–257
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fullard, J.H., Morris, G.K. & Mason, A.C. Auditory processing in the black-sided meadow katydidConocephalus nigropleurum (Orthoptera: Tettigoniidae). J. Comp. Physiol. 164, 501–512 (1989). https://doi.org/10.1007/BF00610444
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00610444