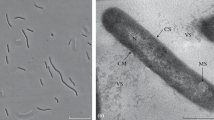
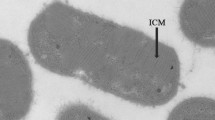
Abstract
The isolation and characterization of a new methanogen from a peat bog, Methanobacterium palustre spec. nov., strain F, is described. Strain F grew on H2/CO2 and formate in complex medium. It also grew autotrophically on H2/CO2. Furthermore, growth on 2-propanol/CO2 was observed. Methane was formed from CO2 by oxidation of 2-propanol to acetone or 2-butanol to 2-butanone, but growth on 2-butanol plus CO2 apparently was too little to be measurable. Similarly, Methanobacterium bryantii M. o. H. and M. o. H. G formed acetone and 2-butanone from 2-propanol and 2-butanol, but no growth was measurable.
On the basis of morphological and biochemical features strain F could be excluded from the genus Methanobrevibacter. Due to its cell morphology, lipid composition and polyamine pattern it belonged to the genus Methanobacterium. From known members of this genus strain F could be distinguished either by a different G+C content of the DNA, low DNA-DNA homology with reference strains, lacking serological reactions with anti-S probes and differences in the substrate spectrum.
An alcohol dehydrogenase activity, specific for secondary alcohols and its substrate specificity was determined in crude extracts of strain F. NADP+ was the only electron carrier that was utilized. No reaction was found with NAD+, F420, FMN and FAD.
Similar content being viewed by others
Abbreviations
- NAD+ :
-
nicotinamide adenine dinucleotide
- NADH2 :
-
reduced form of NAD+
- NADP+ :
-
nicotinamide adenine dinucleotide phosphate
- NADPH2 :
-
reduced form of NADP+
- FMN:
-
flavin adenine mononucleotide
- FAD:
-
flavin adenine dinucleotide
- ADH:
-
alcohol dehydrogenase
- F420 :
-
8-hydroxy-7,8-didemethyl-5-deazaflavin
- SSC:
-
standard saline citrate (0.15 M NaCl, 0.015 M trisodium citrate, pH 7.5)
References
Aranki A, Freter R (1972) Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr 25:1329–1334
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: Reevaluation of a unique biological group. Microbiol Rev 43:260–296
Bligh EG, Dyer WJ (1959) A rapid method of lipid extraction and purification. Can J Biochem Physiol 37:911–917
Blotevogel K-H, Fischer U (1985) Isolation and characterization of a new thermophilic and autotrophic methane producing bacterium: Methanobacterium thermoaggregans spec. nov. Arch Microbiol 142:218–222
Blotevogel K-H, Fischer U, Mocha M, Jannsen S (1985) Methanobacterium thermoalcaliphilum spec. nov., a moderately alcaliphilic and thermophilic autotrophic methanogen. Arch Microbiol 142:211–217
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bryant F, Ljungdahl LG (1981) Characterization of an alcohol dehydrogenase from Thermoanaerobacter ethanolicus active with ethanol and secondary alcohols. Biochem Biophys Res Commun 100:793–799
Bryant MP, Boone D (1987) Isolation and characterization of Methanobacterium formicicum MF. Int J Syst Bacteriol 37:171
Bryant MP, Wolin EA, Wolin MJ, Wolfe RS (1967) Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol 59:20–31
Collins MD, Lund BM, Farrow JAE, Schleifer KH (1983) Chemotaxonomic study of an alkalophilic bacterium, Exiguobacterium aurantiacum gen. nov., sp. nov. J Gen Microbiol 129:2037–2042
DeLey J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Eirich LD, Vogels GD, Wolfe RS (1979) Distribution of coenzyme F420 and properties of its hydrolytic fragments. J Bacteriol 140:20–27
George HA, Johnson JL, Moore WEC, Holdeman LV, Chen JS (1983) Acetone, isopropanol, and butanol production by Clostridium beijerinckii (syn. Clostridium butylicum) and Clostridium aurantibutyricum. Appl Environ Microbiol 45:1160–1163
Grant WD, Pinch G, Harris JE, De Rosa M, Gambacorta A (1985) Polar lipids in methanogen taxonomy. J Gen Microbiol 131: 3277–3286
Hiu SF, Zhu C-X, Yan R-T, Chen J-S (1987) Butanol-ethanol dehydrogenase and butanol-ethanol-isopropanol dehydrogenase: different alcohol dehydrogenases in two strains of Clostridium beijerinckii (Clostridium butylicum). Appl Environ Microbiol 53:697–703
Hus VAR, Fest H, Schleifer K-H (1983) Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol 4:184–192
Jain MK, Thompson TE, Conway de Macario E, Zeikus JG (1987) Speciation of Methanobacterium strain Ivanov as Methanobacterium ivanovii, sp. nov. Syst Appl Microbiol 9:77–82
Kates M (1978) Techniques of lipidology: isolation, analysis and identification of lipids. In: Burdon RH, van Knippenberg PH (eds) Laboratory techniques in biochemistry and molecular biology, vol 3. Elsevier, Amsterdam New York Oxford, pp 1–464
Kneifel H, Stetter KO, Andreesen JR, Wiegel J, König H, Schoberth SM (1986) Distribution of polyamines in representative species of archaebacteria. Syst Appl Microbiol 7:241–245
König H (1984) Isolation and characterization of Methanobacterium uliginosum sp. nov. from a marshy soil. Can J Microbiol 30:1477–1481
Koga Y, Ohga M, Nishihara M, Morii H (1987) Distribution of a diphytanyl ether analog of phosphatidylserine and an ethanolamine containing tetraether lipid in methanogenic bacteria. Syst Appl Microbiol 9:176–182
Kutzenok A, Aschner M (1952) Degenerative processes in a strain of Clostridium butylicum. J Bacteriol 64:829–836
Lamed RJ, Zeikus JG (1981) Novel NADP-linked alcohol-aldehyde, ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J 195:183–190
Langlykke AF, Peterson WH, Fred EB (1937) Reductive processes of Clostridium butylicum and the mechanism of formation of isopropyl alcohol. J Bacteriol 34:443–453
Macario AJL, Conway de Macario E (1983) Antigenic fingerprinting of methanogenic bacteria with polyclonal antibody probes. Syst Appl Microbiol 4:451–458
Macario AJL, Conway de Macario E (1985) Monoclonal antibodies of predefined molecular specificity for identification and classification of methanogens and for probing their ecological niches. In: Macario AJL, Conway de Macario E (eds) Monoclonal antibodies against bacteria, vol II. Academic Press, Orlando, Florida, pp 213–247
Marmur J, Doty P (1962) Determinations of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Minnikin DE, Alashamaony L, Goodfellow M (1975) Differentiation of Mycobacterium, Nocardia, and related taxa by thin layer chromatography analysis of whole-organism methanolysates. J Gen Microbiol 88:200–204
Minnikin DE, Collins MD, Goodfellow M (1979) Fatty acid and polar lipid composition in the classification of Cellulomonas, Oerskovia, and related taxa. J Appl Bacteriol 47:87–95
Nishihara M, Koga Y (1987) Extraction and composition of polar lipids from the archaebacterium Methanobacterium thermoautotrophicum: effective extraction of tetraether lipids by an acidified solvent. J Biochem 101:997–1005
Platen H, Schink B (1987) Methanogenic degradation of acetone by an enrichment culture. Arch Microbiol 149:136–141
Rella R, Raja CA, Pensa M, Pisani FM, Gambacorta A, De Rosa M, Rossi M (1987) A novel archaebacterial NAD+-dependent alcohol dehydrogenase. Eur J Biochem 167:475–479
Scherer P, Kneifel H (1983) Distribution of polyamines in methanogenic bacteria. J Bacteriol 154:1315–1322
Tindall BJ (1985) Qualitative and quantitative distribution of diether lipids in haloalkaliphilic archaebacteria. Syst Appl Microbiol 6:243–246
Widdel F (1986) Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl Environ Microbiol 51:1056–1062
Winter J (1983) Maintenance of stock cultures of methanogens in the laboratory. Syst Appl Microbiol 4:558–563
Winter J, Lerp C, Zabel H-P, Wildenauer FX, König H, Schindler F (1984) Methanobacterium wolfei sp. nov., a new tungstenrequiring, thermophilic, autotrophic methanogen. Syst Appl Microbiol 5:457–466
Worakit S, Boone D, Mah RA, Abdel-Samie M-E, El-Hawagi MM (1986) Methanobacterium alcaliphilum sp. nov., an H2-utilizing methanogen that grows at high pH values. Int J Syst Bacteriol 36:380–382
Zeikus JG (1980) Chemicals and fuel production by anaerobic bacteria. Ann Rev Microbiol 34:423–464
Zeikus JG, Wolfe RS (1972) Methanobacterium thermoautotrophicum sp. nov., an anaerobic, autotrophic, extreme thermophile. J Bacteriol 109:707–713
Zellner G, Winter J (1987a) Secondary alcohols as hydrogen donors for CO2-reduction by methanogens. FEMS Microbiol Lett 44:323–328
Zellner G, Winter J (1987b) Analysis of a highly efficient methanogenic consortium producing biogas from whey. Syst Appl Microbiol 9:284–292
Zellner G, Alten C, Stackebrandt E, Conway de Macario E, Winter J (1987) Isolation and characterization of Methanocorpusculum parvum spec. nov., a new tungsten requiring, coccoid methanogen. Arch Microbiol 147:13–20
Zhilina TN, Ilarisnov SA (1985) Characteristics of formate assimilating methane bacteria and description of Methanobacterium thermoformicicum sp. nov., an anaerobic, autotrophic, extreme thermophile. Microbiology (Engl. Translation of Mikrobiologiya) 53:647–651
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zellner, G., Bleicher, K., Braun, E. et al. Characterization of a new mesophilic, secondary alcohol-utilizing methanogen, Methanobacterium palustre spec. nov. from a peat bog. Arch. Microbiol. 151, 1–9 (1988). https://doi.org/10.1007/BF00444660
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00444660