Summary
The role of parasites in the evolution of host reproductive modes has gained renewed interest in evolutionary ecology. It was previously argued that obligate parthenogenesis (all-female reproduction) arose in a freshwater snail, Campeloma decisum, as a consequence of severe sperm limitation caused by an unencysted trematode, Leucochloridiomorpha constantiae. In the present study, certain conditions are examined for parasitic castration to account for the maintenance of parthenogenesis: the spatial patterns of the prevalence and intensity of infection on a broad geographical scale and its relationship to host genotype; the recovery from infection after isolation from sources of infection; age-related patterns of infections; and the effects of L. constantiae on snail fecundity.
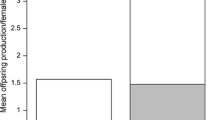
In contrast to the common pattern of the aggregated distribution of parasites within host populations, many snail populations with high prevalence and intensity of infection have non-aggregated parasite distributions. Clonal genotype of the host explained little of the variation in intensity and prevalence of infection by the parasite. Female snails maintained similar prevalence and intensity of infection after isolation, and individuals accumulated parasites throughout their lifespan, both of which suggest there is no effective immune response to infection by L. constantiae. Snail fecundity is not significantly influenced by the intensity of infection. These results suggest that L. constantiae may have represented a strong selective force against males during the initial introduction of this parasite into sexual snail populations because of the persistent nature of infection.
Similar content being viewed by others
References
Allison LN (1943) Leucochloridiomorpha constantiae (Mueller) (Brachylaemidae), its life cycle and taxonomic relationships among digenetic trematodes. Trans Am Microsc Soc 62:127–168
Anderson RM, May RM (1979) Prevalence of schistosome infection witin molluscan populations: observed patterns and theoretical predictions. Parasitology 79:63–94
Anderson RM, Crombie J (1984) Experimental, studies of ageprevalence curves for Schistosoma mansoni infections in populations of Biomphalaria glabrata. Parasitology 89:79–104
Bell G (1982) The Masterpiece of Nature: The Evolution and Genetics of Sexuality. The University of California Press, Berkeley
Bell, G (1985) Two theories of sex and variation. Experientia 41:1235–1245
Bell, G (1988) Uniformity and diversity in the evolution of sex. In: Michod RE, Levin BR (eds) The evolution of sex: An examination of current ideas. Sinauer, Sunderland, MA., pp 126–138
Bierzychude KP (1985) Patterns in plant parthenogenesis. Experientia 41:1255–1264
Bremermann HJ (1980) Sex and polymorphism as strategies in host-pathogen interactions. J Theor Biol 87:671–702
Bremermann, HJ (1985) The adaptive significance of sexuality. Experientia 41:1245–1254
Burch JB (1989) North American freshwater snails. Malacological Publications. Hamburg, Michigan, USA
Crofton HD (1971) A quantitative approach to parasitism. Parasitology 62:179–193
Elliott JM (1971) Some methods for the statistical analysis of samples of benthic invertebrates. Freshwater Biological Association, Scientific Publication no. 25
Feinberg SE (1987) The analysis of cross-classified categorical data. The MIT Press, Cambridge, Massachusetts
Glesener RR, Tilman D (1978) Sexuality and the components of environmental uncertainty: Clues from geographic parthenogenesis in terrestrial animals. Am Nat 112:659–673
Hamilton, WD (1980) Sex versus non-sex versus parasite. Oikos 35:282–290
Hamilton WD (1982) Pathogens as causes of genetic diversity in their host populations. In: Anderson RM, May RM (eds) Population biology of infectious diseases. Springer, Berlin Heidelberg New York Tokyo, pp 363–381
Jaenike J (1978) An hypothesis to account for the maintenance of sex within populations. Evol Theor 3:191–194
Johnson SG (In press) Spontaneous and hybrid origins of apomictic parthenogenesis in a freshwater snail. Heredity
Karlin AA, Vail VA, Heard WH (1980) Parthenogenesis and biochemical variation in southeastern Campeloma geniculum (Gastropoda: Viviparidae). Malacological Review 13:7–15
Levin D (1975) Pest pressure and recombination systems in plants. Am Nat 109:437–451
Lively CM (1987) Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature 328519–521
Lively CM (1989) Adaptation by a parasitic trematode to local populations of its snail host. Evolution 43:1663–1671
Lively CM, Craddock C, Vrijenhoek RC (1990) Red Queen hypothesis supported by parasitism in sexual and clonal fish. Nature 344:864–866
May RM (1977) Togetherness among schistosomes: its effects on the dynamics of infection. Mathem Biosc 35:301–343
Maynard Smith J (1976) A short term advantage for sex and recombination through sib-competition. J Theor Biol 63:245–258
Maynard Smith J (1978) The Evolution of Sex. Cambridge University Press, Cambridge
Moritz C, McCallum H, Donnellan S, Roberts JD (1991) Parasite loads in parthenogenetic and sexual lizard (Heteronotia binoei): support for the Red Queen hypothesis. Proc R Soc Lond B 244:145–149
Mulvey M, Goater TM, Each GW, Crews A (1987) Genotype frequency differences in Halipegus occidualis-infected and unifected Helisoma anceps. J Parasitol 73:757–761
Pennycuick L (1971) Frequency distribution of parasites in a population of three-spined sticklebacks, Gasterosteus aculeatus L., with particular reference to the negative binomial distribution. Parasitology 63:389–406
Richards CS (1975) Genetic factors in susceptibility of Biomphalaria glabrata for different strains of Schistosoma mansoni. Parasitology 70:231–241
SAS Institute (1985) SAS user's guide: statistics. SAS Institute, Inc. Cary, North Carolina
Seger, J and WD Hamilton, 1988. Parasites and sex, In: Michod RE, Levin BR (eds) The Evolution of Sex: An Examination of Current Ideas. Sinauer, Sutherland, MA, pp 176–193
Selander RK, Smith MH, Yang SY, Johnson WE, Gentry JB (1971) Biochemical polymorphism and systematics in the genus Peromyscus. I. Variability in the old-field mouse (Peromyscus polionotus). University of Texas Publications 7103:49–90
Selander RK, Parker ED, Browne RA (1977) Clonal variation in the parthenogenetic snail, Campeloma decisa (Viviparidae). The Veliger 20:349–351
Stearns SC (1987). The evolution of sex and its consequences. In: Stearns, S (ed) The evolution of sex and its consequences. Birkhauser Verlag, Basel, pp 15–31
van der Schalie H (1965) Observation on the sex of Campeloma (Gastropoda: Viviparidae). Occ Pap Mus Zool Univ Mich 641:1–15
Woolhouse MEJ (1989) On the interpretation of age-prevalence curves for schistosome infections of host snails. Parasitology 99:47–56
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Johnson, S.G. Parasite-induced parthenogenesis in a freshwater snail: stable, persistent patterns of parasitism. Oecologia 89, 533–541 (1992). https://doi.org/10.1007/BF00317160
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317160