Abstract
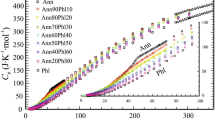
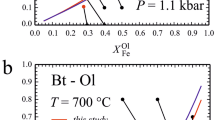
The existing experimental data [Ferry and Spear 1978; Perchuk and Lavrent'eva 1983] on Mg−Fe partitioning between garnet and biotite are disparate. The underlying assumption of ideal Mg−Fe exchange between the minerals has been examined on the basis of recently available thermochemical data. Using the updated mixing parameters for the pyrope-almandine asymmetric regular solution as inputs [Ganguly and Saxena 1984; Hackler and Wood 1984], thermodynamic analysis points to non-ideal mixing in the phlogopite-annite binary in the temperature range of 550°C–950°C. The non-ideality can be approximated by a temperature-independent, one constant Margules parameter. The retrieved values for enthalpy of mixing for Mg−Fe biotites and the standard state enthalpy and entropy changes of the exchange reaction were combined with existing thermochemical data on grossular-pyrope and grossular-almandine binaries to obtain geothermometric expressions for Mg−Fe fractionation between biotite and garnet. [T in K]
The reformulated geothermometer is an improvement over existing biotite-garnet geothermometers because it reconciles the experimental data sets on Fe−Mg partitioning between the two phases and is based on updated activity-composition relationship in Fe−Mg−Ca garnet solid solutions.
Similar content being viewed by others
References
Bhattacharya A, Krishnakumar K, Raith M, Sen SK (1991) An improved set of a-X parameters for pyrope-almandine binary and refinement of orthopyroxene-garnet thermometer and orthopyroxene-garnet-plagioclase-quartz barometer. J Petrol 32:629–656
Davies OL, Goldsmith PL (1986) Statistical methods in research and production. Longman, London
Davis JC (1986) Statistics and data analysis in Geology. John Wiley and Sons, New York
Dymek RF (1983) Titanium, aluminium, and interlayer cation substitutions in biotite from high grade gneiss, west Greenland. Am Mineral 68:880–889
Ferry JM, Spear FS (1978) Experimental calibration of the partitioning of Fe and Mg between biotite and garnet. Contrib Mineral Petrol 66:113–117
Ganguly J, Saxena SK (1984) Mixing properties of aluminosilicate garnets: constraints from natural and experimental data, and application to geothermo-barometry. Am Mineral 69:88–97
Geiger CA, Newton RC, Kleppa OJ (1987) Enthalpy of mixing of synthetic almandine-grossular and almandine-pyrope garnets from high temperature solution calorimetry. Geochim Cosmochim Acta 51:1755–1763
Hackler RT, Wood BJ (1989) Experimental determination of Fe and Mg exchange between garnet and olivine and estimation of Fe−Mg garnet mixing properties. Am Mineral 74:994–999
Indares A, Martignole J (1985) Biotite-garnet geothermometry in the granulite facies: the influence of Al and Ti in biotite. Am Mineral 70:272–278
Kawasaki T, Matsui Y (1983) Thermodynamic analyses of equilibria involving olivine, orthopyroxene and garnet. Geochim Cosmochim Acta 47:1661–1679
Korzhinskii DS (1959) Physico-chemical basis of the analysis of the paragensis of minerals. Trans Consultants Bureau, New York
Lee SM, Ganguly J (1988) Equilibrium compositions of coexisting garnet and orthopyroxene: experimental determinations in the system FeO−MgO−Al2O3 SiO2, and applications. J Petrol 29:93–113
Newton RC, Haselton HT (1981) Thermodynamics of the garnet-plagioclase-Al2SiO5-quartz geobarometer. In: Newton RC, Navrotsky A, Wood BJ (eds) Thermodynamics of minerals and melts. Springer, New York, pp. 129–145
Newton RC, Charlu TV, Kleppa OJ (1980) Thermochemistry of the high state plagioclases. Geochim Cosmochim Acta 44:933–941
Perchuk LL, Lavrent'eva IV (1983) Experimental investigation of exchange equilibria in the system cordierite-garnet-biotite. In: Saxena SK (ed) Kinetics and equilibrium in mineral reactions. Springer, New York, pp 199–239
Pownceby MI, Wall VJ, O'Neill ASC (1987) Fe−Mn partitioning between garnet and ilmenite: experimental calibration and applications. Contrib Mineral Petrol 97:116–126
Sengupta, P, DasGupta S, Bhattacharya PK, Hariya Y (1989) Mixing behaviour in quaternary garnet solid solution and an extended Ellis and Green garnet-clinopyroxene geothermometer. Contrib Mineral Petrol 103:223–227
Thompson AB (1976) Mineral reactions in pelitic rocks. I. Prediction of P-T-X(Fe−Mg) phase relations. II. Calculations of some P-T-X(Fe−Mg) phase relations. Am J Sci 276:401–454
Wohl, K (1953) Thermodynamic evaluation of binary and ternary liquid systems. Chem Eng Prog 49:218–219
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bhattacharya, A., Mohanty, L., Maji, A. et al. Non-ideal mixing in the phlogopite-annite binary: constraints from experimental data on Mg−Fe partitioning and a reformulation of the biotite-garnet geothermometer. Contrib Mineral Petrol 111, 87–93 (1992). https://doi.org/10.1007/BF00296580
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00296580