Summary
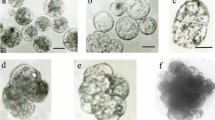
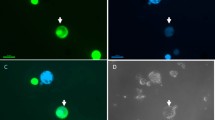
An efficient procedure for obtaining somatic hybrids between B. oleracea and B. campestris has been developed. Hypocotyl protoplasts of B. oleracea were fused with mesophyll protoplasts from three different varieties of B. campestris by the polyethylene glycoldimethylsulfoxide method. The selection of somatic hybrids utilized the inactivation of B. oleracea protoplasts by iodoacetamide (IOA) and the low regeneration ability of B. campestris. The efficiency of recovery of somatic hybrids depended upon the IOA concentration, and when 15 mM IOA was used, 90% of the regenerated plants were found to be hybrid. The somatic hybrids were examined for i) leaf morphology, ii) leucine aminopeptidase (LAP) isozyme and iii) chromosome number. All the hybrids had intermediate leaf morphology and possessed LAP isozymes of both parental species. The chromosome analysis revealed a considerable variation in chromosome number of somatic hybrids, showing the occurrence of multiple fusion and chromosome loss during the culture. Some of the hybrids flowered and set seeds.
Similar content being viewed by others
References
Arús P, Orton TJ (1983) Inheritance and linkage relationships of isozyme loci in Brassica oleracea. J Hered 74:405–412
Belliard G, Vedel F, Pelletier G (1979) Mitochondrial recombination in cytoplasmic hybrids of Nicotiana tabacum by protoplast fusion. Nature 281:401–403
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res 50:151–158
Glimelius K (1984) High growth rate and regeneration capacity of hypocotyl protoplasts in some Brassicaceae. Physiol Plant 61:38–44
Hanson MR, Rothenberg M, Boeshore ML, Nivison HT (1985) Organelle segregation and recombination following protoplast fusion: analysis of sterile cytoplasms. In: Zaitlin M et al. (eds) Biotechnology in plant science. Academic Press, London New York, pp 129–144
Harms CT (1983) Somatic incompatibility in the development of higher plant somatic hybrids. Quart Rev Biol 58:325–353
Haydu Z, Lazar G, Dudits D (1977) Increased frequency of polyethylene glycol induced protoplast fusion by dimethylsulfoxide. Plant Sci Lett 10:357–360
Kameya T, Horn ME, Widholm JM (1981) Hybrid shoot formation from fused Daucus carota and D. capillifolius protoplasts. Z Pflanzenphysiol 104:459–466
Kao KN, Michayluk MK (1975) Nutritional requirements for growth of Vinca hajastana cells and protoplasts at a very low population density in liquid media. Planta 126:105–110
Medgyesy P, Fejes E, Maliga P (1985) Interspecific chloroplast recombination in a Nicotiana somatic hybrid. Proc Natl Acad Sci USA 82:6960–6964
Menczel L, Wolfe K (1984) High frequency of fusion induced in freely suspended protoplast mixtures by polyethylene glycol and dimethylsulfoxide at high pH. Plant Cell Rep 3:196–198
Nehls R (1978) The use of metabolic inhibitors for the selection of fusion products in higher plant protoplasts. Mol Gen Genet 166:117–118
Nishibayashi S, Kaeriyama J (1986) Structural stability of chromosomes in rice (Oryza sativa L.) plants regenerated from somatic tissue culture. Plant Tissue Culture Lett 3:31–34
Nitch C, Nitch JP (1967) The induction of flowering in vitro in stem segment of Plambago indica L. 1. The production of vegetative buds. Planta 72:355–370
Nitch C, Nitch JP (1969) Haploid plants from pollen grains. Science 163:85–87
Pelletier G, Primard C, Vedel F, Chetrit P, Remy R, Rousselle, Renard M (1983) Intergeneric cytoplasmic hybridization in Cruciferae by protoplast fusion. Mol Gen Genet 191:244–250
Schenck HR, Röbbelen G (1982) Somatic hybrids by fusion of protoplasts from Brassica oleracea and B. campestris. Z Pflanzenzücht 89:278–288
Sidorov VA, Menczel L, Nagy F, Maliga P (1981) Chloroplast transfer in Nicotiana based on metabolic complementation between irradiated and iodoacetate treated protoplasts. Planta 152:341–345
Sundberg E, Glimelius K (1986) A method for production of interspecific hybrids within Brassiceae via somatic hybridization, using resynthesis of Brassica napus as a model. Plant Sci 43:155–162
Widholm JM (1982) Selection of protoplast fusion hybrids. In: Fujiwara A (ed) Plant tissue culture. Maruzen, Tokyo 1982, pp 609–612
Yamashita Y, Shimamoto K (1986) Plant regeneration from cabbage protoplasts. In: Bajaj VPS (ed) Biotechnology of crop improvement. Springer, Berlin Heidelberg New York Tokyo (in press)
Author information
Authors and Affiliations
Additional information
Communicated by K.Tsunewaki
Rights and permissions
About this article
Cite this article
Terada, R., Yamashita, Y., Nishibayashi, S. et al. Somatic hybrids between Brassica oleracea and B. campestris: selection by the use of iodoacetamide inactivation and regeneration ability. Theoret. Appl. Genetics 73, 379–384 (1987). https://doi.org/10.1007/BF00262504
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00262504