Abstract
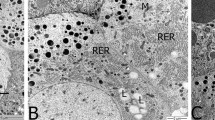
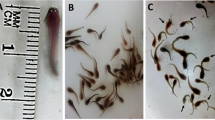
Adult male whitethroated munias, Lonchura malabarica (Aves; Passeriformes), were orally administered with methyl parathion (O, O-dimethyl O-(4-nitrophenyl) phosphorothioate), an extensively used organophosphate pesticide, in graded sublethal dose (5 μg-, or 10 μg-, or 20 μg/100 g body wt/day) for variable durations (1-, 5-, or 10 day/s) during their peak reproductive activities in an annual gonadal cycle. No subtle changes in the feeding behavior, mobility, and body weight were noted between the control and different groups of pesticide-fed birds. As a result of the treatment, the paired testicular weight became reduced significantly only after 10 days at 10 μg- and 20 μg dose levels, but significant decrease in the number of tubules containing healthy germ cells occurred even after single administration of methyl parathion (MP) at the lowest dose (5 μg/100 g). With the increase in dose and progress of treatment, the number of tubules with healthy germ cells became gradually decreased. The activity of acetylcholinesterase (AChE) in both the brain and testes of MP-treated birds was inhibited in a dose and duration dependent manner. A significant negative correlation was observed between the number of tubules containing degenerated germ cells in the testis and the AChE activity in both the brain and testes of MP-administered birds. However, no remarkable changes in the cytomorphological features, including the nuclear diameter of Leydig cells, were noted in any testis of the pesticide-treated munias. The results of the present investigation suggest that methyl parathion ingestion is harmful to male gametogenic functions in the studied passeriform bird, and the given pesticide may exert its antigonadal effect by impairing cholinergic functions of the brain and/or testes.
Similar content being viewed by others
References
Amann RP (1982) A critical review of methods for evaluation of spermatogenesis from seminal characteristics. J Androl 2:37–58
Anam KK, Maitra SK (1995) Impact of quinalphos on blood glucose and acetylcholinesterase (AChE) activity in brain and pancreas in a roseringed parakeet (Psittacula krameri borealis: Newmann). Arch Environ Contam Toxicol (in press)
Bhatnagar P, Soni I (1990) Toxic effects of phosphamidon on the testes of Swiss albino mice. Bull Environ Contam Toxicol 45:590–597
Boekelheide K, Diana Neely M, Sioussat TM (1989) The Sertoli cell cytoskeleton: A target for toxicant induced germ cell loss. Toxicol Appl Pharmacol 101:373–389
Chakraborty J, Nelson L (1976) Comparative study of cholinesterases distribution in the spermatozoa of some mammalian species. Biol Reprod 15:579–585
Chapin RE, Lamb JC IV (1984) Effects of ethylene glycol monoethyl ether on various parameters of testicular function in the F 344 rat. Environ Health Perspectives 57:219–224
Chapin RE, Phelps JL, Somkuti SG, Heindel JJ, Burka LT (1990) The interaction of Sertoli and Leydig cells in the testicular toxicity of tri-O-cresyl phosphate. Toxicol Appl Pharmacol 104:483–495
Christensen AK (1975) Leydig cells. In: Hamilton DW, Greep RO (eds) Handbook of physiology, Vol. V. American Physiological Society, Washington, DC, pp 57–94
Civen M, Brown CB, Morin RJ (1977) Effects of organophosphate insecticides on adrenal cholesteryl ester and steroid metabolism. Biochem Pharmacol 26:1901–1907
Crews D, Silver R (1985) Reproductive physiology and behaviour interactions in nonmammalian vertebrates. In: Adler N, Pfaff D, Goy RW (eds) Handbook of behavioural neurobiology, Vol. VII. Plenum Publishing Corporation, pp 101–182
Ellman GL, Courtney D, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fowler DL, Mahan JN (1980) The pesticide review. Agric. Stabilization Conserv. Ser., USDA, Washington, DC
Ghosh A, Banerjee J (1983) Effect of population stress on the histopathology of avian endocrine organs. J Yamashina Inst Ornithol 15:156–166
Goodman DR, Harbison RD (1981) CHaracterization of enzymatic acetylcholine synthesis by mouse brain, rat sperm, and purified carnitine acetylcholinesterase. Biochem Pharmacol 30:1521–1528
Grue CE, Fleming WJ, Busby DG, Hill EF (1983) Assessing hazards of organophosphate pesticides to wildlife. Trans North Amer Wildl Nat Resour Conf 48:200–220
Grue CE, Shipley BK (1984) Sensitivity of nestling and adult starlings to dicrotophos, an organophosphate pesticide. Environ Res 35:454–465
Hanna PJ, Kerr JB (1981) Antifertility activity of trimethylphosphate in male rats. Experientia 37:999–1001
Heywood R, James RW (1985) Current laboratory approaches for assessing male reproductive toxicity: Testicular toxicity in laboratory animals. In: Dixon RL (ed) Reproductive toxicology. Raven Press, NY, pp 147–160
Hill EF, Heath RG, Spann JW, Williams JD (1975) Lethal dietary toxicities of environmental pollutants to birds. US Fish and Wildlife Service Spec Science Rep Wildlife, No. 191, Washington, DC, p 61
Holmes SB, Boag PT (1990) Effects of the organophosphorus pesticide fenitrothion on behavior and reproduction in zebra finches. Environ Res 53:62–75
Ishii S, Yamamoto K (1976) Demonstration of follicle stimulating hormone (FSH) activity in hypophyseal extracts of various vertebrates by the response of Sertoli cells of the chick. Gen Comp Endocrinol 29:506–510
Krause W, Hamm K, Weissmuller J (1976) Damage to spermatogenesis in juvenile rat treated with DDVP and malathion. Bull Environ Contam Toxicol 15:458–462
Lee KP, Kinney LA, Valentine R (1989) Comparative testicular toxicity of bis (2-methoxyethyl) ether and 2-methoxyethanol in rats. Toxicology 59:239–258
Maitra SK (1986) Annual testicular cycle of blossomheaded parakeet, Psittacula cyanocephala (Aves: Psittacidae), under natural environmental conditions. J Interdiscipl Cycle Res 17:213–223
— (1987) Influences of length of photoperiod on the testicular activity of the blossomheaded parakeet, Psittacula cyanocephala. J Yamashina Inst Ornithol 19:28–44
Maitra SK, Sarkar R (1991) Histopathological changes in the testes after oral administration of quinalphos, an organosphosphorus pesticide, in a subtropical wild bird Psittacula krameri. Eur Arch Biol (Bruxelles) 102:413–429
—, — (1993) Evaluation of testicular responsiveness to ingestion of methyl parathion in roseringed parakeets (Psittacula krameri Neumann). Pest Res J 5:60–67
Rattner BA, Eroschenko VP, Fox GA, Fry DM, Gorsline J (1984) Avian endocrine responses to environmental pollutants. J Exptl Zool 232:683–689
Rattner BA, Sileo L, Scanes CG (1982) Oviposition and the plasma concentrations of LH, progesterone, and corticosterone in bobwhite quail (Colinus virginianus) fed parathion. J Reprod Fertil 66:147–155
Rattner BA, Robert NC, Ottinger MA (1986) Depression of plasma luteinizing hormone concentration in quail by the anticholinesterase insecticide parathion. Comp Biochem Physiol C 83:451–453
Ray A, Chatterjee S, Bagchi P, Das TK, Deb C (1987) Effect of quinalphos on testicular steroidogenesis in rats. Andrologia 19:163–168
Sarkar R, Maitra SK (1989) Testicular responses to phosphamidon, an organophosphate pesticide, in a wild bird Psittacula krameri. Arch Biol (Bruxelles) 100:459–468
—, — (1990) Responses of adrenal medulla to oral administration of organophosphate pesticide in roseringed parakeets Psittacula krameri borealis (Neumann). Eur Arch Biol (Bruxelles) 101:469–480
Somkuti SG, Lapadula DM, Chapin RE, Lamb JC IV, Abou-Donia MB (1987) Testicular toxicity following oral administration of tri-o-cresyl phosphate (TOCP) in roosters. Toxicol Lett 37:279–290
Stone WB, Gradoni PB (1985) Recent poisoning of wild birds by diazinon and carbofuran. Northeastern Environ Sci 4:160–164
Tanabe Y, Ogawa T, Nakamura T (1981) The effects of short-term starvation on pituitary and plasma LH, plasma estradiol and progesterone, and on pituitary response to LH-RH in the laying hen (Gallus domesticus). Gen Comp Endocrinol 43:392–398
Weiss B (1988) Behavior as an early indicator of pesticide toxicity. Toxicol Industrial Health 4:351–360
Zar JH (1974) Biostatistical analysis. Prentice-Hall, London
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maitra, S.K., Sarkar, R. Influence of methyl parathion on gametogenic and acetylcholinesterase activity in the testis of whitethroated munia (Lonchura malabarica) . Arch. Environ. Contam. Toxicol. 30, 384–389 (1996). https://doi.org/10.1007/BF00212298
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00212298