Abstract
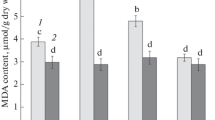
Effects of flooding on the activities of some enzymes of activated oxygen metabolism, the levels of antioxidants, and lipid peroxidation in senescing leaves of tobacco were investigated. As judged by the decrease in chlorophyll and protein levels, flooding accelerated the senescence of tobacco leaves. Total peroxide and the lipid peroxidation product, malondialdehyde, increased in both control and flooding-treated leaves with increasing duration of the experiment. Throughout the duration of the experiment, flooded leaves had higher levels of total peroxide and malondialdehyde than did control leaves. Flooding resulted in an increase in peroxidase and ascorbate peroxidase activities and a reduction of superoxide dismutase activity in the senescing leaves. Glycolate oxidase, catalase, and glutathione reductase activities were not affected by flooding. Flooding increased the levels of total ascorbate and dehydroascorbate. Total glutathione, reduced form glutathione, or oxidized glutathione levels in flooded leaves were lower than in control leaves during the first two days of the experiment, but were higher than in control leaves at the later stage of the experiment. Our work suggests that senescence of tobacco induced by flooding may be a consequence of lipid peroxidation possibly controlled by superoxide dismutase activity. Our results also suggest that increased rates of hydrogen peroxide in leaves of flooded plants could lead to increased capacities of the scavenging system of hydrogen peroxide.
Similar content being viewed by others
Abbreviations
- GSH:
-
reduced form glutathione
- GSSG:
-
oxidized form glutathione
- GSSG reductase:
-
glutathione reductase
- MDA:
-
malondialdehyde
- SOD:
-
superoxide dismutase
References
Brennan T, Rychter A, Frenkel C (1979) Activity of enzymes involved in the turnover of hydrogen peroxide during fruit senescence. Bot Gaz 140: 384–388
Chowdhury SR and Choudhuri MA (1985) Hydrogen peroxide metabolism as an index of water stress tolerance in jute. Physiol Plant 65: 503–507
Curtis CR (1971) Disc electrophoretic comparisons of proteins and peroxidases from Phaseolus vulgaris leaves infected with Agrobacterium tumefaciens. Can J Bot 49: 333–337
Dhindsa RS and Reid DM (1982) Leaf senescence and lipid peroxidation: Effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol Plant 56: 453–457
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 126: 93–101
Elstner EF (1982) Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol 33: 73–96
Foster JG and Hess JL (1980) Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66: 482–487
Gillham DJ and Dodge AD (1984) Some effects of paraquat on plants. Plant Physiol 75: S-51
Goyal A (1987) Effect of water stress on glycolate metabolism in the leaves of rice seedlings (Oryza sativa). Physiol Plant 69: 289–294
Gross GG, Jansh C, Elstner EF (1977) Involvement of malate, monophenols, and the superoxide radical in hydrogen peroxide formation by isolated cell walls from horseradish (Amoracia lapathifolia Gilib.). Planta 136: 271–276
Heath RL and Packer L (1968) Photoperoxidation in isolated chloroplasts. 1. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125: 189–198
Hunter MIS, Hetherington AM, Crawford RMM (1983) Lipid peroxidation — a factor in anoxia intolerance in Iris species? Phytochemistry 22: 1145–1147
Hurng WP and Kao CH (1993) Growth response of tobacco to flooding. Bot Bull Acad Sin 34 (In press)
Kato M and Shimuzu S (1987) Chlorophyll metabolism in higher plants VII. Chlorophyll degradation in senescring tobacco leaves: phenolic-dependent peroxidative degradation. Can J Bot 65: 729–735
Kellogg EW and Fridovich I (1975) Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem 250: 8812–8817
Kozlowski TT (1984) Plant responses to flooding of soil. BioScience 34: 162–167
Laws MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach chloroplasts: the effect of hydrogen peroxide and of paraquat. Biochem J 210: 899–903
Leshem YY (1981) Oxy free radicals and plant senescence. What's New Plant Physiol 12: 1–4
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Mondal R and Choudhuri MA (1981) Role of hydrogen peroxide in senescence of excised leaves of rice and maize. Biochem Physiol Pflanz 176: 700–709
Mondal R and Choudhuri MA (1982) Regulation of senescence of excised leaves of some C3 and C4 species by endogenous H2O2. Biochem Physiol Pflanz 177: 403–417
Mondal R and Choudhuri MA (1984) Interaction of phytohormones with H2O2 metabolism and senescence of excised leaves of some C3 and C4 plants. Biochem Physiol Pflanz 179: 463–471
Monk LS, Fagerstedt KV, Crawford RMM (1989) Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiol Plant 76: 456–459
Mukherjee SP and Choudhuri MA (1983) Implication of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58: 166–170
Nakano Y and Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22: 867–880
Parida RK, Kar M, Mishra D (1978) Enhancement of senescence in excised rice leaves by hydrogen peroxide. Can J Bot 56: 2937–2941
Pauls KR and Thompson JE (1984) Evidence for the accumulation of peroxidized lipids in membranes of senescing cotyledons. Plant Physiol 75: 1152–1157
Pedersen TC and Aust SD (1973) The role of superoxide and singlet oxygen in lipid peroxidation promoted by xanthine oxidase. Biochem Biophys Res Commun 52: 1071–1078
Polle A, Krings B, Ronnenberg H (1989) Superoxide dismutase activity in needles of Norwegian spruce tree (Picea abies L.). Plant Physiol 90: 1310–1315
Price ML and Butler LG (1977) Rapid visual estimation and spectrometric determination of tannin content of sorghum grain. J Agric Food Chem 25: 1268–1273
Sagisaka S (1976) The occurrence of peroxide in a perennial plant, Populus gelrica. Plant Physiol 57: 308–309
Shigeoka S, Onishi T, Nakano Y, Kitaoka S (1987) Photo-induced biosynthesis of glutathione in Euglena. Agric Biol Chem 43: 2053–2058
Shigeoka S, Yokota A, Nakano Y and Kitaoka S (1979) The effect of illumination on the L-ascorbic acid in Euglena gracilis Z. Agric Biol Chem 43: 2053–2058
Smirnoff N and Colombe SV (1988) Drought influences the activity of enzymes of the chloroplast hydrogen peroxide scavenging system, J Exp Bot 39: 1097–1108
Smith IK (1985) Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol 79: 1044–1047
Tanaka K, Suda Y, Kondo N, Sugahara K (1985) O3 tolerance and the ascorbate-dependent H2O2 decomposing system in chloroplasts. Plant Cell Physiol 26: 1425–1431
Thomas H and Stoddart JL (1981) Leaf senescence. Annu Rev Plant Physiol 31: 83–111
Ushimaru T, Shibasaka M, Tsujii H (1992) Development of the O2-detoxification system during adaptation to air of submerged rice seedlings. Plant Cell Physiol 33: 1065–1071
Van Toai TT and Bolles CS (1991) Postanoxic injury in soybean (Glycine max) seedlings. Plant Physiol 97: 588–592
Wintermans JFGM and De Mots A (1965) Spectrophotometric characteristic of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta 109: 448–453
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hurng, W.P., Kao, C.H. Effect of flooding on the activities of some enzymes of activated oxygen metabolism, the levels of antioxidants, and lipid peroxidation in senescing tobacco leaves. Plant Growth Regul 14, 37–44 (1994). https://doi.org/10.1007/BF00024139
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00024139