Abstract
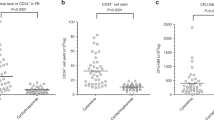
The CTD (cyclophosphamide, thalidomide, and dexamethasone) regimen is known to be an effective primary therapy in patients with newly diagnosed multiple myeloma (MM). However, stem cell yields after CTD remain inconsistent. The aim of the present study is to identify the influence of the CTD regimen on the outcome of peripheral blood stem cell (PBSC) collection. Fifty-four patients received four cycles of CTD, and PBSCs were mobilized with cyclophosphamide and G-CSF or with G-CSF alone. Each patient from whom ≤4.0 × 106 CD34+ cells/kg were collected received a second mobilization course. The median duration from the start of a CTD regimen to the first collection was 4.3 months. Forty-eight patients were mobilized with cyclophosphamide followed by G-CSF, and six patients were mobilized with G-CSF alone. The median day of apheresis was day 3 (range day 2–day 5). The overall response rate at mobilization was 96.3 %, including 11.1 % complete response, 22.2 % very good partial response, and 63.0 % partial response. The median number of harvested CD34+ cells was 12.8 × 106 cells/kg. At the second mobilization, 88.9 % of patients reached the minimal stem cell collection target of ≥2.0 × 106 cells/kg, and 75.9 % of patients achieved the collection target of ≥4.0 × 106 cells/kg. CTD within four cycles is an effective primary therapy in patients with newly diagnosed MM and only minimally affects subsequent PBSC collection.


Similar content being viewed by others
References
Kristinsson SY, Landgren O, Dickman PW, Derolf AR, Bjorkholm M. Patterns of survival in multiple myeloma: a population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25:1993–9.
Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–6.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60.
Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114:1729–35.
Kim JE, Yoo C, Kim S, Lee DH, Kim SW, Lee JS, et al. Optimal timing of G-CSF administration for effective autologous stem cell collection. Bone Marrow Transplant. 2011;46:806–12.
Cavallo F, Bringhen S, Milone G, Ben-Yehuda D, Nagler A, Calabrese E, et al. Stem cell mobilization in patients with newly diagnosed multiple myeloma after lenalidomide induction therapy. Leukemia. 2011;25:1627–31.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–42.
Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P, et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15:718–23.
Koh KR, Janz M, Mapara MY, Lemke B, Stirling D, Dorken B, et al. Immunomodulatory derivative of thalidomide (IMiD CC-4047) induces a shift in lineage commitment by suppressing erythropoiesis and promoting myelopoiesis. Blood. 2005;105:3833–40.
Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–6.
Cavo M, Zamagni E, Tosi P, Tacchetti P, Cellini C, Cangini D, et al. Superiority of thalidomide and dexamethasone over vincristine–doxorubicin–dexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106:35–9.
Sidra G, Williams CD, Russell NH, Zaman S, Myers B, Byrne JL. Combination chemotherapy with cyclophosphamide, thalidomide and dexamethasone for patients with refractory, newly diagnosed or relapsed myeloma. Haematologica. 2006;91:862–3.
Yang DH, Kim YK, Sohn SK, Chung JS, Joo YD, Lee JH, et al. Induction treatment with cyclophosphamide, thalidomide, and dexamethasone in newly diagnosed multiple myeloma: a phase II study. Clin Lymphoma Myeloma Leuk. 2010;10:62–7.
Morgan GJ, Davies FE, Gregory WM, Russell NH, Bell SE, Szubert AJ, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118:1231–8.
Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Navarro Coy N, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97(3):442–50.
Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–39.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Eng J Med. 1996;335:91–7.
Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–41.
Breitkreutz I, Lokhorst HM, Raab MS, Holt B, Cremer FW, Herrmann D, et al. Thalidomide in newly diagnosed multiple myeloma: influence of thalidomide treatment on peripheral blood stem cell collection yield. Leukemia. 2007;21:1294–9.
Auner HW, Mazzarella L, Cook L, Szydlo R, Saltarelli F, Pavlu J, et al. High rate of stem cell mobilization failure after thalidomide and oral cyclophosphamide induction therapy for multiple myeloma. Bone Marrow Transplant. 2011;46:364–7.
Lefrere F, Zohar S, Ghez D, Delarue R, Audat F, Suarez F, et al. The VAD chemotherapy regimen plus a G-CSF dose of 10 µg/kg is as effective and less toxic than high-dose cyclophosphamide plus a G-CSF dose of 5 µg/kg for progenitor cell mobilization: results from a monocentric study of 82 patients. Bone Marrow Transplant. 2006;37:725–9.
Morris CL, Siegel E, Barlogie B, Cottler-Fox M, Lin P, Fassas A, et al. Mobilization of CD34+ cells in elderly patients (≥70 years) with multiple myeloma: influence of age, prior therapy, platelet count and mobilization regimen. Br J Haematol. 2003;120:413–23.
Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8.
Sharabi A, Haran-Ghera N. Immune recovery after cyclophosphamide treatment in multiple myeloma: implication for maintenance immunotherapy. Bone Marrow Res. 2011;2011:269519.
Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8.
Gertz MA, Kumar SK, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43:619–25.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jung, SH., Park, H., Ahn, JS. et al. Efficacy of stem cell mobilization in patients with newly diagnosed multiple myeloma after a CTD (cyclophosphamide, thalidomide, and dexamethasone) regimen. Int J Hematol 97, 92–97 (2013). https://doi.org/10.1007/s12185-012-1237-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1237-0