Abstract
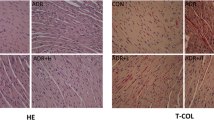
Carfilzomib is a proteasome inhibitor, commonly used in multiple myeloma, but its clinical use may be limited due to cardiotoxicity. This study was aimed to evaluate the influence of rutin in carfilzomib-induced cardiotoxicity in rats. Wistar albino male rats weighing 200–250 g (approximately 10 weeks old) were taken for this study. Animals were divided into four groups of six animals each. Group 1 served as normal control (NC), received normal saline; group 2 animals received carfilzomib (dissolved in 1 % DMSO) alone; group 3 animals received rutin (20 mg/kg) + carfilzomib; and group 4 animals received rutin (40 mg/kg) + carfilzomib. Hematological changes, biochemical changes, oxidative stress, hypertrophic gene expression, apoptotic gene expression, NFκB and IκB-α protein expression and histopathological evaluation were done to confirm the finding of carfilzomib-induced cardiotoxicity. Treatment with rutin decreased the carfilzomib-induced changes in cardiac enzymes such as lactate dehydrogenase, creatine kinase (CK) and CK-MB. For the assessment of cardiotoxicity, we further evaluated cardiac hypertrophic gene and apoptotic gene expression such as α-MHC, β-MHC and BNP and NF-κB and p53 gene expression, respectively, using RT-PCR. Western blot analysis showed that rutin treatment prevented the activation of NF-κB by increasing the expression of IκB-α. Rutin also attenuated the effects of carfilzomib on oxidant-antioxidant including malondialdehyde and reduced glutathione. Histopathological study clearly confirmed that rutin attenuated carfilzomib-induced cardiotoxicity in rats.








Similar content being viewed by others
References
Demo, S. D., Kirk, C. J., Aujay, M. A., Buchholz, T. J., Dajee, M., Ho, M. N., et al. (2007). Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Research, 67, 6383–6391.
Hajek, R., Bryce, R., Ro, S., Klencke, B., & Ludwig, H. (2012). Design and rationale of FOCUS (PX-171-011): A randomized, open-label, phase 3 study of carfilzomib versus best supportive care regimen in patients with relapsed and refractory multiple myeloma (R/R MM). BMC Cancer, 12, 415–521.
Herndon, T. M., Deisseroth, A., Kaminskas, E., Kane, R. C., Koti, K. M., Rothmann, M. D., et al. (2013). Food and drug administration approval: Carfilzomib for the treatment of multiple myeloma. Clinical Cancer Research, 19(17), 4559–4563.
Fuchs, O., Provaznikova, D., Marinov, I., Kuzelova, K., & Spicka, I. (2009). Antiproliferative and proapoptotic effects of proteasome inhibitors and their combination with histone deacetylase inhibitors on leukemia cells. Cardiovascular & Hematological Disorders: Drug Targets, 9, 62–77.
Khan, R. Z., & Badros, A. (2012). Role of carfilzomib in the treatment of multiple myeloma. Expert Review of Hematology, 5, 361–372.
Vij, R., Siegel, D. S., Jagannath, S., Jakubowiak, A. J., Stewart, A. K., McDonagh, K., et al. (2012). An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. British Journal of Haematology, 158, 739–748.
Chari, A., & Hajje, D. (2014). Case series discussion of cardiac and vascular events following carfilzomib treatment: Possible mechanism, screening, and monitoring. BMC Cancer, 14, 915–923.
Siegel, D., Martin, T., Nooka, A., Harvey, R. D., Vij, R., Niesvizky, R., et al. (2013). Integrated safety profile of single-agent carfilzomib: Experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica, 98, 1753–1761.
Siegel, D. S., Martin, T., Wang, M., Vij, R., Jakubowiak, A. J., Lonial, S., et al. (2012). A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood, 120(14), 2817–2825.
Force, W. I. T. (1980). Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. British Heart Journal 44(6), 672–673.
Abelmann, W. H. (1984). Classification and natural history of primary myocardial disease. Progress in Cardiovascular Diseases, 27(2), 73–94.
Richardson, P., McKenna, W., Bristow, M., Maisch, B., Mautner, B., O’Connell, J., et al. (1996). Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation, 93(5), 841–842.
Shanmugarajan, T. S., Arunsunder, M., Somasundaram, I., Krishnakumar, E., Sivaraman, D., & Ravichandiran, V. (2008). Protective effect of Ficus hispida Linn. on cyclophosphamide provoked oxidative myocardial injury in rat model. International Journal of Pharmacology, 4(2), 78–87.
Repetto, A., Dal Bello, B., Pasotti, M., Agozzino, M., Vigano, M., Klersy, C., et al. (2005). Coronary atherosclerosis in end-stage idiopathic dilated cardiomyopathy: An innocent bystander? European Heart Journal, 26(15), 1519–1527.
Barry, S. P., Davidson, S. M., & Townsend, P. A. (2008). Molecular regulation of cardiac hypertrophy. International Journal of Biochemistry & Cell Biology, 40(10), 2023–2039.
Miyata, S., Minobe, W., Bristow, M. R., & Leinwand, L. A. (2000). Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circulation Research, 86(4), 386–390.
Reiser, P. J., Portman, M. A., Ning, X. H., & Schomisch Moravec, C. (2001). Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. American Journal of Physiology Heart and Circulatory Physiology, 280(4), H1814–H1820.
Lee, H., Son, C. B., Shin, S. H., & Kim, Y. S. (2008). Clinical correction between brain natriuretic peptide and anthracycline-induced cardiotoxicity. Cancer Research and Treatment, 40, 121–126.
Cowie, M. R., Jourdain, P., Maisel, A., Dahlstrom, U., Follath, F., Isnard, R., et al. (2003). Clinical applications of B-type natriuretic peptide (BNP) testing. European Heart Journal, 24(19), 1710–1718.
Oeckinghaus, A., & Ghosh, S. (2009). The NF-κB family of transcription factors and its regulation. Cold Spring Harbor Perspectives in Biology, 1(4), 1–14.
Liu, S. F., & Malik, A. B. (2006). NF-kappa B activation as a pathological mechanism of septic shock and inflammation. American Journal of Physiology. Lung Cellular and Molecular Physiology, 290(4), L622–L645.
Brandes, R. P., & Kreuzer, J. (2005). Vascular NADPH oxidases: Molecular mechanisms of activation. Cardiovascular Research, 65, 16–27.
Konukoglu, D., Serin, O., Kemerli, D. G., Serin, E., Hayirhoglu, A., & Oner, B. (1998). A study on the carotid artery intima-media thickness and its association with lipid peroxidation. Clinica Chimica Acta, 277, 91–98.
Inoue, M. (2011). Protective mechanisms against reactive oxygen species. In I. M. Arias, J. L. Boyer, N. Fausto, W. B. Jokoby, D. A. Schachter, & D. A. Shafritz (Eds.), The liver: Biology and pathobiology (5th ed., pp. 443–459). New York: Raven Press.
Wu, G., Fang, Y. Z., Yang, S., Lupton, J. R., & Turner, N. D. (2004). Glutathione metabolism and its implications for health. Journal of Nutrition, 134, 489–492.
Zindenberg, C. S., Olin, K. L., & Villarweva, J. (1991). Ethanol induced changes in hepatic free radical defense mechanisms and fatty acid composition in the miniature pig. Hepatology, 13, 1185–1192.
Altinterim, B. (2014). Citrus, rutin and on their vein permeability effects. RJAEM, 3(2), 80–81.
Heather, S., Demrow, B. S., Peter, R., Slane, B. S., & John, D. F. (1995). Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. American Heart Association, 91, 1182–1188.
Benavente-Garcia, O., & Castillo, J. (2008). Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and antiinflammatory activity. Journal of Agriculture and Food Chemistry, 56(15), 6185–6205.
Panchal, S. K., Poudyal, H., Arumugam, T. V., & Brown, L. (2011). Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. Journal of Nutrition, 141, 1062–1069.
Panchal, S. K., Poudyal, H., & Brown, L. (2012). Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in DIET-induced metabolic syndrome in rats. Journal of Nutrition, 142(6), 1026–1032.
Yang, J., Wang, Z., Fang, Y., Jiang, J., Zhao, F., Wong, H., et al. (2011). Pharmacokinetics, pharmacodynamics, metabolism, distribution, and excretion of carfilzomib in rats. Drug Metabolism and Disposition, 39, 1873–1882.
Imam, F., Al-Harbi, N. O., Al-Harbi, M. M., Ansari, M. A., Zoheir, K. M., Iqbal, M., et al. (2015). Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacological Research, 102, 1–11.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Korashy, H. M., & El-Kadi, A. O. (2004). Differential effects of mercury, lead and copper on the constitutive and inducible expression of aryl hydrocarbon receptor (AHR)-regulated genes in cultured hepatoma Hepa 1c1c7 cells. Toxicology, 201(1–3), 153–172.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.
Sedlak, J., & Lindsay, R. H. (1968). Estimation of total, protein bound and non-protein bound sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochemistry, 25, 192–205.
Al-Harbi, N. O., Imam, F., Nadeem, A., Al-Harbi, M. M., Iqbal, M., Rahman, S., et al. (2014). Protection against tacrolimus-induced cardiotoxicity in rats by olmesartan and aliskiren. Toxicology Mechanisms and Methods, 24(9), 697–702.
Singal, P. K., & Iliskovic, N. (1998). Doxorubicin-induced cardiomyopathy. New England Journal of Medicine, 339, 900–905.
Yeh, E. T. H., Tong, A. T., Lenihan, D. J., Yusuf, S. W., Swafford, J., Champion, C., et al. (2004). Review: Current perspective: Cardiovascular complications of cancer therapy diagnosis, pathogenesis, and management. Circulation, 109, 3122–3131.
Al-Shabanah, O., Aleisa, A. M., Hafez, M. M., Al-Rejaie, S. S., Al-Yahya, A. A., Bakheet, S. A., et al. (2012). Desferrioxamine attenuates doxorubicin-induced acute cardiotoxicity through TFG-β/Smad p53 pathway in rat model. Oxidative Medicine and Cellular Longevity, 2012, 1–7.
Piura, B., & Rabinovich, A. (2005). Doxorubicin and ifosfamidemesna in advanced and recurrent uterine sarcomasl. European Journal of Gynaecological Oncology, 26(3), 275–278.
Al-Shabanah, O., Mansour, M., El-Kashef, H., & Al-Bekairi, A. (1998). Captopril ameliorates myocardial and hematological toxicities induced by adriamycin. Biochemistry and Molecular Biology International, 45, 419–427.
el-Missiry, M. A., Othman, A. I., Amer, M. A., & Abdel-Aziz, M. A. (2001). Attenuation of the acute adriamycin-induced cardiac and hepatic oxidative toxicity by N-(2-mercaptopropionyl) glycine in rats. Free Radical Research, 35, 575–581.
Rashikh, A., Najmi, A. K., Akhtar, M., Mahmood, D., Pillai, K. K., & Ahmad, S. J. (2011). Protective effects of aliskiren in doxorubicin-induced acute cardiomyopathy in rats. Human and Experimental Toxicology, 30, 102–109.
Yagmurca, M., Fadillioglu, E., Erdogan, H., Ucar, M., Sogut, S., & Irmak, M. K. (2003). Erdosteine prevents doxorubicin-induced cardiotoxicity in rats. Pharmacological Research, 48, 377–382.
Korashy, H. M., Al-Suwayeh, H. A., Maayah, Z. H., Ansari, M. A., Ahmad, S. F., & Bakheet, S. A. (2015). Mitogen-activated protein kinases pathways mediate the sunitinib-induced hypertrophy in rat cardiomyocyte H9c2 cells. Cardiovascular Toxicology, 15(1), 41–51.
Maayah, Z. H., Ansari, M. A., El Gendy, M. A., Al-Arifi, M. N., & Korashy, H. M. (2014). Development of cardiac hypertrophy by sunitinib in vivo and in vitro rat cardiomyocytes is influenced by the aryl hydrocarbon receptor signaling pathway. Archives of Toxicology, 88(3), 725–738.
Das, B., Young, D., Vasanji, A., Gupta, S., Sarkar, S., & Sen, S. (2010). Influence of p53 in the transition of myotrophin-induced cardiac hypertrophy to heart failure. Cardiovascular Research, 87(3), 524–534.
Surget, S., Khoury, M. P., & Bourdon, J. C. (2013). Uncovering the role of p53 splice variants in human malignancy: A clinical perspective. OncoTargets and Therapy, 7, 57–68.
Cusack, J. C., Liu, R., & Baldwin, A. S. (1999). NF-kappa B and chemoresistance: Potentiation of cancer drugs via inhibition of NF-kappa B. Drug Resistance Updates, 2(4), 271–273.
Tergaonkar, V., Pando, M., Vafa, O., Wahl, G., & Verma, I. (2002). p53 stabilization is decreased upon NFkappaB activation: A role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell, 1(5), 493–503.
Perkins, N. D., & Gilmore, T. D. (2006). Good cop, bad cop: the different faces of NF-kappaB. Cell Death and Differentiation, 13(5), 759–772.
Ahmad, S. F., Attia, S. M., Bakheet, S. A., Zoheir, K. M. A., Ansari, M. A., Korashy, H. M., et al. (2015). Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of NF-κb, STAT3 and pro-inflammatory mediators and enhancement of IκBα and anti-inflammatory cytokines. Inflammation, 38(2), 846–857.
Ibrahim, M. A., Ashour, O. M., Ibrahim, Y. F., El-Bitar, H. I., Gomaa, W., & Abdel-Rahim, S. R. (2009). Angiotensin-converting enzyme inhibition and angiotensin AT(1)-receptor antagonism equally improve doxorubicin-induced cardiotoxicity and nephrotoxicity. Pharmacological Research, 60, 373–381.
Acknowledgments
The present work was funded by King Saud University, Deanship of Scientific Research, College of Pharmacy (Project No. RGP-VPP-305). The authors acknowledge the Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University for its facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Imam, F., Al-Harbi, N.O., Al-Harbia, M.M. et al. Rutin Attenuates Carfilzomib-Induced Cardiotoxicity Through Inhibition of NF-κB, Hypertrophic Gene Expression and Oxidative Stress. Cardiovasc Toxicol 17, 58–66 (2017). https://doi.org/10.1007/s12012-015-9356-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-015-9356-5