Abstract
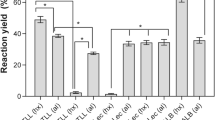
A new assay was designed to measure the release of omega-3 acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] from the hydrolysis of sardine oil by lipases immobilized inside porous supports. A biphasic system was used containing the fish oil dissolved in the organic phase and the immobilized lipase suspended in the aqueous phase. The assay was optimized by using a very active derivative of Rhizomucor miehei lipase (RML) adsorbed onto octyl-Sepharose. Standard reaction conditions were: (a) an organic phase composed by 30/70 (v:v) of oil in cyclohexane, (b) an aqueous phase containing 50 mM methyl-cyclodextrin in 10 mM Tris buffer at pH 7.0. The whole reaction system was incubated at 25 °C. Under these conditions, up to 2% of the oil is partitioned into the aqueous phase and most of the 95% of released acids were partitioned into the organic phase. The organic phase was analyzed by RP-HPLC (UV detection at 215 nm) and even very low concentrations (e.g., 0.05 mM) of released omega-3 fatty acid could be detected with a precision higher than 99%. Three different lipases adsorbed on octyl-Sepharose were compared: Candida antarctica lipase-fraction B (CALB), Thermomyces lanuginosa lipase (TLL) and RML. The three enzyme derivatives were very active. However, most active and selective towards polyunsaturated fatty acids (PUFA) versus oleic plus palmitic acids (a fourfold factor) was CALB. On the other hand, the most selective derivatives towards EPA versus DHA (a 4.5-fold factor) were TLL and RML derivatives.




Similar content being viewed by others
References
Fernandez L, Banuelos O, Zafra A, Ronchel C, Perez-Victoria I, Morales JC, Velasco J, Adrio JL (2008) Alteration of substrate specificity of Galactomyces geotrichum BT107 lipase I on eicosapentaenoic acid-rich triglycerides. Biocatal Biotransform 26:296–305
Heird WC (2001) The role of polyunsaturated fatty acids in term and preterm infants and breastfeeding mothers. Pediatr Clin North Am 48:173–188
Demaison L, Moreau D (2002) Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: a possible mechanism of action. Cell Mol Life Sci 59:463–477
Saremi A, Arora R (2009) The utility of omega-3 fatty acids in cardiovascular disease. Am J Ther 16:421–436
Antypa N, Van Der Does AJW, Smelt AHM, Rogers RD (2009) Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J Psychopharmacol 23:831–840
Bougnoux P, Hajjaji N, Maheo K, Couet C, Chevalier S (2010) Fatty acids and breast cancer: sensitization to treatments and prevention of metastatic re-growth. Prog Lipid Res 49:76–86
Montgomery P, Richardson AJ (2008) Omega-3 fatty acids for bipolar disorder. Cochrane Database of Systematic Reviews, 2
Siddiqui RA, Harvey KA, Zaloga GP (2008) Modulation of enzymatic activities by n-3 polyunsaturated fatty acids to support cardiovascular health. J Nutr Biochem 19:417–437
GISSI-HF Investigators (2008) Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372:1223–1230
Riediger ND, Othman RA, Suh M, Moghadasian MH (2009) A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc 109:668–679
Mateo C, Palomo JM, Fuentes M, Betancor L, Grazu V, López-Gallego F, Pessela BCC, Hidalgo A, Fernández-Lorente G, Fernández-Lafuente R, Guisán JM (2006) Glyoxyl agarose: a fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzyme Microb Technol 39:274–280
Bastida A, Sabuquillo P, Armisen P, Fernández-Lafuente R, Huguet J, Guisán JM (1998) A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol Bioeng 58:486–493
Godoy CA, Fernández-Lorente G, Felice M, De las Rivas B, Guisán JM, Palomo JM (2010) Dramatic increase in activity of immobilized derivatives of lipase from Geobacillus thermocatenolatus. Synergistic effect of different additives and site-directed chemical modification. J Mol Catal B: Enzym (in press)
Rodrigues RC, Bolivar JM, Volpato G, Filice M, Godoy C, Fernandez-Lafuente R, Guisan JM (2009) Improved reactivation of immobilized-stabilized lipase from Thermomyces lanuginosus by its coating with highly hydrophilic polymers. J Biotechnol 144:113–119
Loftsson T, Brewster ME (1996) Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci 85:1017–1025
Strickley RG (2004) Solubilizing excipients in oral and injectable formulations. Pharm Res 21:201–230
Fava F, Bertin L, Fedi S, Zannoni D (2003) Methyl-β-cyclodextrin-enhanced solubilization and aerobic biodegradation of polychlorinated biphenyls in two aged-contaminated soils. Biotechnol Bioeng 81(4):381–390
Fava F, Ciccotosto VF (2002) Effects of randomly methylated-β-cyclodextrins (RAMEB) on the bioavailability and aerobic biodegradation of polychlorinated biphenyls in three pristine soils spiked with a transformer oil. Appl Microb Biotechnol 58(3):393–399
Cui Y, Wang C, Mao J, Yu Y (2010) A facile and practical approach to randomly methylated β-cyclodextrin. J Chem Technol Biotechnol 85:248–251
Palomo JM, Muñoz G, Fernández-Lorente G, Mateo C, Fernández-Lafuente R, Guisán JM (2002) Interfacial adsorption of lipases on very hydrophobic support (octadecyl-Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J Mol Catal B 19:279–286
Fernandez-Lafuente R, Armisén P, Sabuquillo P, Fernández-Lorente G, Guisán JM (1998) Immobilization of lipases by selective adsorption on hydrophobic supports. Chem Phys Lipids 93:185–197
Aucoin MG, Erhardt FA, Legge RL (2004) Hyperactivation of Rhizomucor miehei lipase by hydrophobic xerogels. Biotechnol Bioeng 85:647–655
Gámez-Meza N, Higuera-Ciapara I, Calderon De La Barca AM, Vázquez-Moreno L, Noriega-Rodríguez J, Angulo-Guerrero O (1999) Seasonal variation in the fatty acid composition and quality of sardine oil from Sardinops sagax caeruleus of the Gulf of California. Lipids 34:639–642
Rombaut R, De Clercq N, Foubert I, Dewettinck K (2009) Triacylglycerol analysis of fats and oils by evaporative light scattering detection. J Am Oil Chem Soc 86(1):19–25
Fernandez-Lorente G, Cabrera Z, Godoy C, Fernandez-Lafuente R, Palomo JM, Guisan JM (2008) Interfacially activated lipases against hydrophobic supports: effect of the support nature on the biocatalytic properties. Process Biochem 43:1061–1067
Kanicky JR, Shah DO (2002) Effect of degree, type, position of unsaturation on the pKa of long-chain fatty acids. J Colloid Interface Sci 256(1):201–207
Bes MT, Gomez-Moreno C, Guisan JM, Fernandez-Lafuente R (1995) Selective oxidation: stabilisation by multipoint attachment of ferredoxin NADP + reductase, an interesting cofactor recycling enzyme. J Mol Catal A 98:161–169
Betancor L, López-Gallego F, Hidalgo A, Alonso-Morales N, Fuentes M, Fernández-Lafuente R, Guisán JM (2004) Prevention of interfacial inactivation of enzymes by coating the enzyme surface with dextran-aldehyde. J Biotechnol 110:201–207
Godoy C, de las Rivas B, Filice M, Fernández-Lorente G, Guisan JM, Palomo, JM (2009) Enhanced activity of an immobilized lipase promoted by site- directed chemical modification with polymers. Process Biochem 45:534–541
Acknowledgments
This work was sponsored by the Spanish Ministry of Science and Innovation (project AGL-2009-07526) and the Comunidad Autonoma de Madrid (Project S0505/PPQ/03449). We gratefully recognize the Spanish Ministry of Science and Innovation for the “Ramón y Cajal” contract for Dr. Fernandez-Lorente. We thank the Spanish Ministry of Science and Innovation (MICINN) grant Consolider INGENIO 2010 CSD2007-00063 FUN-C-FOOD and the Comunidad de Madrid (CAM) ALIBIRD-S2009/AGR-1469 for financial support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fernández-Lorente, G., Pizarro, C., López-Vela, D. et al. Hydrolysis of Fish Oil by Lipases Immobilized Inside Porous Supports. J Am Oil Chem Soc 88, 819–826 (2011). https://doi.org/10.1007/s11746-010-1728-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1728-1