Abstract
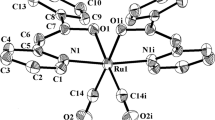
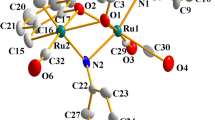
The reactions of Ru3(CO)12 with oximes of α-substituted caran-4-one, lemonen-5- and pinan-3-one derivatives were studied. The reactions at elevated temperatures yield binu-clear complexes Ru2(CO)4L2, with two ruthenium atoms bridging two terpenoid ligands (L) through the oxime groups and coordinated additionally to a nitrogen or sulfur atom of the NR2 or SR groups respectively. The reactions carried out at room temperature in the presense of Me3NO yield trinuclear complexes Ru3(CO)8L2 with analogous coordination of the terpenoid ligands. In a solution at room temperature these clusters readily transform to binuclear complexes. The NMR spectroscopy shows the stereochemical nonrigidity of the complexes with the S-CH2-Ph fragment: at room temperature in a solution the benzyl radical undergoes slow rotation about the S-C bond and a change of the conformation of carane and pinane carbocycles occurs more rapidly. The reactions with pinane and carane derivatives are stereospecific yielding only one of the possible diastereoisomers. More flexible limonene derivative form both diastereoisomers.
Similar content being viewed by others
References
M. Castiglioni, S. Deabate, R. Giordano P. J. King, S. R. Knox, E. Sappa, J. Organomet. Chem., 1998, 571, 251
M. G. Richmond, Coord. Chem. Rev., 2004, 248, 881.
J. Gao, P. Xu, X. Yi, H. Wan, K. Tsai, J. Mol. Catal. A., 1999, 147, 99.
B. Fontal, M. Reyes, T. Suarez, J. Mol. Catal. A., 1999, 149, 75.
Y. Fukuda, K. Kondo, T. Aoyama, Tetrahedron Lett., 2007, 48, 3389
S. Giadiali, S. Medici, T. Kegl, L. Kollar, Monat. Chem., 2000, 131, 1351
J. W. Faller, P. P. Fontaine, J. Organomet. Chem., 2007, 692, 976.
E. V. Gracheva, M. Haukka, B. T. Heaton, E. Norlander, T. A. Pakkanen, S. P. Tunik, Dalton Trans., 2003, 2046
D. V. Krupenya, S. I. Selivanov, S. P. Tunik, M. Haukka, Dalton Trans., 2004, 2541
C. Badij, C. S. Browning, D. Farrar, I. O. Koshevoy, I. S. Podkorytov, A. I. Poe, S. P. Tunik, J. Am. Chem. Soc., 2002, 124, 8922
S. P. Tunik, T. S. Pylyugina, I. O. Koshevoy, S. I. Selivanov, M. Haukka, T. A. Pakkanen, Dalton Trans., 2004, 568.
D. A. Garnovslii, V. Yu. Kukushkin, Uspehi khimii, 2006, 75, 125 [Russ. Chem. Rev, 2006, 75].
V. A. Maksakov, N. V. Pervuhina, V. S. Korenev, N. V. Podberezskaya, V. P. Kirin, A. V. Tkachev, Zhyrn. struct. khim, 2004, 45, 698 [Russ. J. Struct. Chem, 2004, 45].
J. S.-Y. Wong, W.-T. Wong, New J. Chem., 2002, 26, 94.
J. A. Cabeza, I. del Rio, V. Riera, M. Suárez, C. Álvarez-Rúa, S. García-Granda, S. H. Chuang, J. R. Hwu, Eur. J. Inorg. Chem., 2003, 4159.
K.-H. Lee K., W.-T. Wong, J. Chem. Soc., Dalton Trans., 1997, 2987.
D. J. Darensbourg, B. Fontal, S. S. Chojnacki, K. K. Klausmeyer, J. H. Reibenspies, Inorg. Chem., 1994, 33, 3526.
A. J. Deeming, D. W. Owen, N. I. Powell, J. Organomet. Chem., 1990, 398, 299
M.-H. Chao, S. Kumaresan, Y.-S. Wen, S.-C. Lin, J. R. Hwu, K.-L. Lu, Organometallics, 2000, 19, 714
S. Aime, G. Gervasio, L. Milone, R. Rossetti, P. L. Stanghellini, J. Chem. Soc., Dalton Trans., 1978, 534
M. Langenbahn, H. Stoeckli-Evans, G. Süss-Fink, Helv. Chim. Acta, 1991, 74, 549.
A. Bax, J. Magn. Reson., 1983, 53, 517
V. Rutar, J. Magn. Reson., 1984, 58, 306.
A. V. Tkachev, A. V. Rukavishnikov, A. M. Chibiryaev, A. Y. Denisov, Y. V. Gatilov, I. Y. Bagryanskaya, Austr. J. Chem., 1992, 45, 1077.
I. Yu. Shabalina, V. P. Kirin, V. A. Maksakov, A. V. Virivetc, A. V. Golovin, A. M. Agafoncev, A. V. Tkachev, Coord. Khim, 2008, 34, 293 [Russ. J. Coord. Chem. (Engl. Transl.), 2008, 34].
G. M. Sheldrick, SADABS, Program for absorption Correction with the SMART system, University of Göttingen, Germany, 1996.
Bruker (2005b), SHELXTL, Version 6.22, Bruker AXS Inc., Madison, WI, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 7, pp. 1332–1343, July, 2009.
Rights and permissions
About this article
Cite this article
Kirin, V.P., Prikhod’ko, I.Y., Maksakov, V.A. et al. Ruthenium carbonyl complexes with α-substituted oxime derivatives of terpenes as ligands. Russ Chem Bull 58, 1371–1382 (2009). https://doi.org/10.1007/s11172-009-0183-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-009-0183-3