Abstract
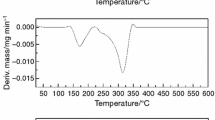
Since administration of capecitabine tablets leading to dose limiting makes the unfavorable toxicity, preparation of sustained-release tablets will overcome most of these side effects. The aim of this study was to prepare and study the stability of capecitabine sustained-release tablets. Sustained-release tablets of capecitabine were characterized by differential scanning calorimetry, X-ray diffraction, and infrared and ultraviolet spectroscopy techniques to determine the stability of the tablets. All tests carried out for tablets upon preparation as well as 6 and 12 months after preparation. The gradual decomposition of capecitabine sustained-release tablets stored at accelerated conditions (40 °C in 75 % of relative humidity) was indicated by decreasing values of peak purity and melting temperature, calculated from the Van’t Hoff equation. Except for the occurrence of one sharp peak for long-term stability and some sharp peaks in the accelerated condition, all peaks showed a crystallized nature. But the FTIR and UV results showed that there were no changes between the initial sustained-release tablets and stored tablets. Although the XRD results showed more peaks in the accelerated condition tablets, the crystalline form of capecitabine was maintained. These findings demonstrate that the capecitabine sustained-release tablet has excellent stability in normal and long-term storage conditions, with slight changes in the accelerated condition.




Similar content being viewed by others
Abbreviations
- μg:
-
Microgram
- ACC:
-
Accelerated time
- API:
-
Active pharmaceutical ingredient
- Cap:
-
Capecitabine
- FDA:
-
Food and drug administration
- Long:
-
Long term
- RH:
-
Relative humidity
- USP:
-
United States of pharmacopeia
- UV:
-
Ultraviolet
References
Łaszcz M, Trzcińska K, Filip K, Szyprowska A, Mucha M, Krzeczyński P. Stability studies of capecitabine. J Therm Anal Calorim. 2011;105(3):1015–21.
Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27(1):23–44. doi:10.1016/j.clinthera.2008.01.00S.
Budman RD. Capecitabine. Invest New Drugs. 2000;18:355–63.
Nutley. Xeloda (capecitabine). Food and Drug Administration (FDA). 2003. http://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf.
Ahmad I, Shaikh RH. Effect of temperature and humidity on hardness and friability of packaged paracetamol tablet formulations. Pak J Pharm Sci. 1994;7(2):69–78.
International Conference on Harmonization (ICH). Guidance for Industry Q1A(R2) Stability Testing of New Drug Substances and Products. Food and Drug Administration. 2003. http://www.fda.gov/cber/gdlns/ichstab.pdf.
Russo Karen A. The role of USP monographs in stability testing. In: Huynh-Ba K, editor. Pharmaceutical Stability Testing to Support Global Markets: Pharmasp. Arlington: American Association of Pharmaceutical Scientists; 2010. p. 51–60.
Agnihotri SA, Aminabhavi TM. Novel interpenetrating network chitosan-poly(ethylene oxide-g-acrylamide) hydrogel microspheres for the controlled release of capecitabine. Int J Pharm. 2006;324(2):103–15.
Gong X, Moghaddam MJ, Sagnella SM, Conn CE, Danon SJ, Waddington LJ, et al. Lyotropic liquid crystalline self-assembly material behavior and nanoparticulate dispersions of a phytanyl pro-drug analogue of capecitabine: a chemotherapy agent. ACS Appl Mater Interfaces. 2011;3(5):1552–61. doi:10.1021/am200117u.
Pare A, Yadav SK, Patil UK. Formulation and evaluation of effervescent floating tablet of amlodipine besylate. Res J Pharm Tech. 2008;1(4):526–30.
Pharmacopoeia British. Uniformity of Content. London: Stationery Office on behalf of the (MHRA); 2010.
Chieng N, Rades T, Aaltonen J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J Pharm Biomed Anal. 2011;55(4):618–44.
Dogra Sanjeev. A chitosan–polymer hydrogel bead system for a metformin HCl controlled release oral dosage form. Toledo: BiblioLabsII; 2011.
Acknowledgements
This study was supported by research grants from IPPP, University of Malaya, Malaysia (Grant No.: PS202/2010B). Also, the authors thank Osvah Pharmaceutical Company, Tehran, Iran for their gift of capecitabine and also to Mrs. Fatemeh Allah Bedashti for her friendship and assistance.
Conflict of interest
There is no conflict of interest in this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davoudi, E.T., Noordin, M.I., Javar, H.A. et al. Stability study of the gastric floating dosage form of capecitabine. J Therm Anal Calorim 115, 2495–2499 (2014). https://doi.org/10.1007/s10973-013-3540-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3540-2