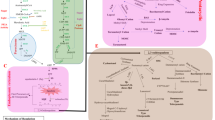
The Pd-catalyzed arylation of isoalantolactone by deoxyvasicinone and lappaconitine halides occurred with formation mainly of cross-coupling products with the (E)-configuration of the double bond. Another three compounds were isolated from the reaction of isoalantolactone with 6-bromodeoxyvasicinone. These were the lactone diarylation product, a compound with the (Z)-configuration of the double bond, and a product with a shift of the C(11,13) double bond and configuration inversion at C(8). The new compounds are interesting as potential biologically active agents.


Similar content being viewed by others
References
J.-M. Gao, W.-J. Wu, J.-W. Zhang, and Y. Konishi, Nat. Prod. Rep., 24, 1153 (2007).
J. W. Blunt, B. R. Copp, W.-P. Hu, M. H. G. Munro, P. T. Northcote, and M. R. Prinsep, Nat. Prod. Rep., 26, 170 (2009).
M. Bruno, S. Rosselli, A. Maggio, R. A. Raccuglia, K. F. Bastow, and K.-H. Lee, Nat. Prod. Rep., 68, 1042 (2005); B. Ding, N. Yin, Y. Liu, J. Cardenas-Garcia, R. Evanson, T. Orsak, M. Fan, G. Turin, and P. B. Savage, J. Am. Chem. Soc., 126, 13642 (2004); Y. Hirokawa, H. Kinoshita, T. Tanaka, K. Nakata, N. Kitadai, K. Fujimoto, S. Kashimoto, T. Kojima, and S. Kato, J. Med. Chem., 51, 1991 (2008); Z. P. Kortylewicz, J. Nearman, and J. Baranowska-Kortylewicz, J. Med. Chem., 52, 5124 (2009).
T. Konishi, Y. Shimada, T. Nagao, H. Okabe, and T. Konoshima, Biol. Pharm. Bull., 25, 1370 (2002); M. T. Lindenmeyer, A. Hrenn, C. Kern, V. Castro, R. Murillo, S. Muller, S. Laufer, J. Schulte-Monting, B. Siedle, and I. Merfort, Bioorg. Med. Chem., 14, 2487 (2006); M.-H. Bang, M.-W. Han, M.-C. Song, J.-G. Cho, H.-G. Chung, T.-S. Jeong, K.-T. Lee, M.-S. Choi, S.-Y. Kim, and N.-I. Baek, Chem. Pharm. Bull., 56, 1168 (2008).
S. Wagner, A. Hofman, B. Siedle, L. Terfloth, I. Merfort, and J. Gasteiger, J. Med. Chem., 49, 2241 (2006).
D.-R. Hwang, Y.-S. Wu, C.-W. Chang, T.-W. Lien, W.-C. Chen, U.-K. Tan, J. T. A. Hsu, and H.-P. Hsieh, Bioorg. Med. Chem., 14, 83 (2006).
S. Zhang, Y. K. Wong, C. N. Ong, and H. M. Shen, Curr. Med. Chem.: Anti-cancer Agents, 5, 239 (2005); M. L. Guzman, R. M. Rossi, L. Karnischky, X. Li, D. R. Peterson, D. S. Howard, and C. T. Jordan, Blood, 105, 4163 (2005).
R. Romagnoli, P. G. Baraldi, M. A. Tabrizi, J. Bermejo, F. Estevez, M. Borgatti, and R. Gambari, J. Med. Chem., 48, 7906 (2005); T. G. Elford, A. Ulaczyk-Lesanko, G. De Pascale, G. D. Wright, D. G. Hall, and J. Gasteiger, J. Comb. Chem., 11, 155 (2009).
A. V. Belovodskii, E. E. Shul’ts, M. M. Shakirov, and G. A. Tolstikov, Dokl. Akad. Nauk, 426, 762 (2009).
A. V. Belovodskii, E. E. Shul’ts, M. M. Shakirov, I. Yu. Bagryanskaya, Yu. V. Gatilov, and G. A. Tolstikov, Zh. Org. Khim., 46, 1532 (2010).
F. Bohlmann, L. N. Dutta, W. Knauf, H. Robinson, and R. M. King, Phytochemistry, 19, 433 (1980).
A. Arcadi, M. Chiarini, F. Marinelli, Z. Berente, and L. Kollar, Org. Lett., 2, 69 (2000).
C. Han, F. J. Barrios, M. V. Riofski, and D. A. Colby, J. Org. Chem., 74, 7176 (2009).
G. B. Shul’pin, Organic Reagents Catalyzed by Metal Complexes [in Russian], Nauka, Moscow, 1988.
S. C. Srivastava, M. M. Mehra, G. K. Trivedi, and S. C. Bhattacharyy, Ind. J. Chem., 9, 512 (1971).
Kh. M. Shakhidoyatov, A. Irisbaev, L. M. Yun, E. Oripov, and Ch. Sh. Kadyrov, Khim. Geterotsikl. Soedin., 1564 (1976).
S. A. Osadchii, E. E. Shul’ts, E. V. Polukhina, M. M. Shakirov, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 1038 (2006).
Acknowledgment
The work was supported financially by the Russian Foundation for Basic Research (Project No. 08-03-00340).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, pp. 747–751, November–December, 2010.
Rights and permissions
About this article
Cite this article
Belovodskii, A.V., Shul’ts, E.E., Shakirov, M.M. et al. Synthesis of hybrid molecules containing fragments of sesquiterpene lactones and plant alkaloids. Chem Nat Compd 46, 880–885 (2011). https://doi.org/10.1007/s10600-011-9774-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-011-9774-y