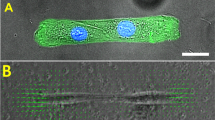
Abstract
Airway smooth muscle cells exhibit stiffening during contractile activation. This stiffening may be interpreted as a result of the stabilizing influence of the mechanical prestress stored within the cytoskeleton (CSK). However, in vivo, airway smooth muscle cells contract while simultaneously experiencing breathing-induced stretching. Excessive stretching of cells could cause actin–myosin crosslinks, and possibly other cytoskeletal filaments, to break, thereby leading to dissipation of the prestress and inhibition of further cell stiffening. The aim of this study is to investigate the stiffening behavior of individual human airway smooth muscle (HASM) cells exposed to a combination of substrate stretching, contractile activation and relaxation. We treated cultured HASM cells with either contractile (histamine) or relaxing (DBcAMP) pharmacological agonists and used magnetic cytometry technique to investigate the stiffening behavior of these cells during uniform substrate stretching (0–30%). Cells that were not treated, as well as those treated with histamine, exhibited increasing stiffening during stretching up to 20% of substrate strain, with additional stiffening becoming inhibited for substrate strains of 20–30%. In contrast, in cells treated with DBcAMP, stretching produced moderate but continuous stiffening with increasing substrate strain. These results indicate that both active and passive components of the prestress contribute to cell stiffening. We also observed that cells permeabilized with saponin exhibited stiffening at low levels (<10%) of substrate stretching, similar to non-permeabilized cells, but not at high levels (10–30%) of stretching, where stiffening was inhibited. These data suggest that at low levels of substrate strains the relative contributions of ion channel activation as well as actin and focal adhesion remodeling are less important for stiffening than passive distension of the CSK. Taken together, our results suggest that both the active and passive components of the cytoskeletal prestress contribute to the stiffening behavior of HASM cells under physiological conditions, but that at high levels of cellular distensions there is a possible tradeoff between these two components with the contribution from the passive component becoming increasingly more important.







Similar content being viewed by others
References
An S. S., Laudadio R. E., Lai J., Rogers R. A., Fredberg J. J. (2002) Stiffness changes in cultured airway smooth muscle cells. Am. J. Physiol. Cell Physiol. 283:C792–C801
Bursac P., Lenormad G., Fabry B., Oliver M., Weitz D. A., Viasnoff V., Butler J. P., Fredberg J. J. (2005) Mechanism unifying cytoskeletal remodeling and slow dynamics in living cells. Nat. Mater. 4:567–561
Chen C. S., Alonso J. L., Ostuni E., Whitesides G. M., Ingber D. E. (2003) Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 307:355–361
Deng L., Fairbank N. J., Fabry B., Smith P. G., Maksym G.N. (2004) Localized mechanical stress induces time-dependent actin cytoskeletal remodelling and stiffening in cultured airway smooth muscle cells Am. J. Physiol. Cell Physiol. 287: C440–C448
Fabry B., Maksym G. N., Butler J. P., Glogauer M., Navajas D., Fredberg J. J. (2001) Scaling the microrheology of living cells. Phys. Rev. Lett. 87:148102
Fabry B., Maksym G. N., Butler J. P., Glogauer M., Navajas D., Taback N. A., Millet E. J., Fredberg J. J. (2003) Time scale and other invariants of integrative mechanical behavior in living cells. Phys. Rev. E 68:041914-1–041914-18
Fabry B., Maksym G. N., Shore S. A., Moore Jr. P. E., Panettieri R. A., Butler J. P., Fredberg J. J. (2001) Signal transduction in smooth muscle selected contribution: Time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J. Appl. Physiol. 91:986–994
Féréol S., Fodil R., Labat B., Galiacy S., Laurent V. M., Louis B., Isabey D. (2006) Sensitivity of alveolar macrophage to substrate mechanical and adhesive properties Cell Motil. Cytoskeleton 63: 321–340
Fredberg J. J., Inouye D., Miller B., Nathan M., Jafari S., Raboudi S. H., Butler J. P., Shore S. A. (1997) Airway smooth muscle, tidal stretch, and dynamically determined contractile stress. Am. J. Respir. Crit. Care Med. 156:1752–1759
Fredberg J. J., Jones K. A., Nathan M., Raboudi S., Prakash Y. S., Shore S. A., Butler J. P., Sieck G. C. (1996) Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J. Appl. Physiol. 81:2703–2712
Fredberg J. J., Stamenović D. (1989) On the imperfect elasticity of lung tissue. J. Appl. Physiol. 67:2408–2419
Galbraith C. G., Yamada K. M., Sheetz M. P. (2002) The relationship between force and focal complex development. J. Cell Biol. 159:695–705
Hirshman C. A., Emala C. W. (1999) Actin organization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am. J. Physiol. Lung Cell Mol. Physiol. 277:L653–L661
Hirshman C. A., Zhu D., Panettieri R. A., Emala C. W. (2001) Actin depolymerization via β-adrenoceptor in airway smooth muscle cells: a novel PKA-independent pathway. Am. J. Physiol. Cell Physiol. 281:C1468–C1476
Hubmayr R. D., Shore S. A., Fredberg J. J., Planus E., Panettieri Jr. R. A., Moller W., Heyder J., Wang N. (1996) Pharmacological activation changes stiffness of cultured human airway smooth muscle cells. Am. J. Physiol. Cell Physiol. 271:C1660–C1668
Ingber D. E. (2003) Cellular tensegrity revisited I. Cell structure and hierarchical systems biology. J. Cell. Sci. 116:1157–1173
Ito S., Majumdar A., Kume H., Shimokata K., Naruse K., Lutchen K. R., Stamenović D., Suki B. (2006) Viscoelastic and dynamic nonlinear properties of airway smooth muscle tissue: roles of mechanical force and the cytoskeleton. Am. J. Physiol. Lung Cell Mol. Physiol. 290:L1227–L1237
Laurent V. M., Fodil R., Cañadas P., Féréol S., Louis B., Planus E., Isabey D. (2003) Partitioning of cortical and deep cytoskeleton responses from transient magnetic bead twisting. Ann. Biomed. Eng. 31:1263–1278
Mehta D., Gunst S. J. (1999) Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J. Physiol. (Lond.) 519:829–840
Mijailovich S. M., Kojic M., Zivkovic M., Fabry B., Fredberg J. J. (2002) A finite element model of cell deformation during magnetic bead twisting. J. Appl. Physiol. 93:1429–1436
Panettieri R. A., Murray R. K., DePalo L. R., Yadvish R. A., Kotlikoff M. I. (1989) A human airway smooth muscle cell line that retains physiological responsiveness. Am. J. Physiol. Cell Physiol. 256:C329–C335
Pourati J., Maniotis A., Spiegel D., Schaffer J. L., Butler J. P, Fredberg J. J., Ingber D. E., Stamenović D., Wang N. (1998) Is cytoskeletal tension a major determinant of cell deformability in adherent endothelial cells? Am. J. Physiol. Cell Physiol. 274:C1283–C1289
Rosenblatt N., Hu S., Chen J., Wang N., Stamenović D. (2004) Distending stress of the cytoskeleton is a key determinant of cell rheological behavior. Biochem. Biophys. Res. Commun. 321:617–622
Sims J. R., Karp D., Ingber D. E. (1992) Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J. Cell Sci. 103:1215–1222
Smith B. A., Tolloczko B., Martin J. G., Grütter P. (2005) Probing the viscoelastic behaviour of cultured airway smooth muscle cells with atomic force microscopy: stiffening induced by contractile agonist. Biophys. J. 88:2994–3007
Stamenović D. (2005) Effects of cytoskeletal prestress on cell rheological behavior. Acta Biomater. 1:255–262
Stamenović D., Wang N. (2000) Invited review: engineering approaches to cytoskeletal mechanics. J. Appl. Physiol. 89:2085–2090
Tang D., Mehta D., Gunst S. J. (1999) Mechanosensitive tyrosine phosphorylation of paxillin and focal adhesion kinase in tracheal smooth muscle. Am. J. Physiol. Cell Physiol. 276:C250–C258
Trepat X., Grabulosa M., Puig F., Maksym G. N., Navajas D., Farré R. (2004) Viscoelasticity of human alveolar epithelial cells subjected to stretch. Am. J. Physiol. Lung Cell Mol. Physiol. 287:L1025–L1034
Wachssotck D. H., Schwartz W. H., Pollard T. D. (1994) Cross-linker dynamics determine the mechanical properties of actin gels. Biophys. J. 66:801–809
Wang N., Ingber D. E. (1994) Control of the cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys. J. 66:2181–2189
Wang N., Naruse K., Stamenović D., Fredberg J. J., Mijailovich S. M., Tolić-Nørrelykke I. M., Polte T., Mannix R., Ingber D. E. (2001) Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA 98:7765–7770
Wang N., Tolić-Nørrelykke I. M., Chen J., Mijailovich S. M., Butler J. P., Fredberg J. J., Stamenović D. (2002) Cell prestress. Stiffness I., and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 282:C606–C616
Acknowledgment
This work was supported by NIH Grants HL-33009 and GM-072744.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenblatt, N., Hu, S., Suki, B. et al. Contributions of the Active and Passive Components of the Cytoskeletal Prestress to Stiffening of Airway Smooth Muscle Cells. Ann Biomed Eng 35, 224–234 (2007). https://doi.org/10.1007/s10439-006-9228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9228-z