Abstract
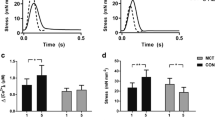
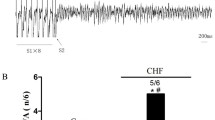
The β1-adrenergic antagonist metoprolol improves cardiac function in animals and patients with chronic heart failure, isolated mitral regurgitation (MR), and ischemic heart disease, though the molecular mechanisms remain incompletely understood. Metoprolol has been reported to upregulate cardiac expression of β3-adrenergic receptors (β3AR) in animal models. Myocardial β3AR signaling via neuronal nitric oxide synthase (nNOS) activation has recently emerged as a cardioprotective pathway. We tested whether chronic β1-adrenergic blockade with metoprolol enhances myocardial β3AR coupling with nitric oxide-stimulated cyclic guanosine monophosphate (β3AR/NO-cGMP) signaling in the MR-induced, volume-overloaded heart. We compared the expression, distribution, and inducible activation of β3AR/NO-cGMP signaling proteins within myocardial membrane microdomains in dogs (canines) with surgically induced MR, those also treated with metoprolol succinate (MR+βB), and unoperated controls. β3AR mRNA transcripts, normalized to housekeeping gene RPLP1, increased 4.4 × 103- and 3.2 × 102-fold in MR and MR+βB hearts, respectively, compared to Control. Cardiac β3AR expression was increased 1.4- and nearly twofold in MR and MR+βB, respectively, compared to Control. β3AR was detected within caveolae-enriched lipid rafts (Cav3+LR) and heavy density, non-lipid raft membrane (NLR) across all groups. However, in vitro selective β3AR stimulation with BRL37344 (BRL) triggered cGMP production within only NLR of MR+βB. BRL induced Ser 1412 phosphorylation of nNOS within NLR of MR+βB, but not Control or MR, consistent with detection of NLR-specific β3AR/NO-cGMP coupling. Treatment with metoprolol prevented MR-associated oxidation of NO biosensor soluble guanylyl cyclase (sGC) within NLR. Metoprolol therapy also prevented MR-induced relocalization of sGCβ1 subunit away from caveolae, suggesting preserved NO-sGC-cGMP signaling, albeit without coupling to β3AR, within MR+βB caveolae. Chronic β1-blockade is associated with myocardial β3AR/NO-cGMP coupling in a microdomain-specific fashion. Our canine study suggests that microdomain-targeted enhancement of myocardial β3AR/NO-cGMP signaling may explain, in part, β1-adrenergic antagonist-mediated preservation of cardiac function in the volume-overloaded heart.







Similar content being viewed by others
References
Aragon JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ (2011) Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol 58:2683–2691. doi:10.1016/j.jacc.2011.09.033
Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ (2006) Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci USA 103:7500–7505
Banquet S, Delannoy E, Agouni A, Dessy C, Lacomme S, Hubert F, Richard V, Muller B, Leblais V (2011) Role of G(i/o)-Src kinase-PI3K/Akt pathway and caveolin-1 in beta(2)-adrenoceptor coupling to endothelial NO synthase in mouse pulmonary artery. Cell Signal 23:1136–1143. doi:10.1016/j.cellsig.2011.02.008
Bartholomeu JB, Vanzelli AS, Rolim NP, Ferreira JC, Bechara LR, Tanaka LY, Rosa KT, Alves MM, Medeiros A, Mattos KC, Coelho MA, Irigoyen MC, Krieger EM, Krieger JE, Negrao CE, Ramires PR, Guatimosim S, Brum PC (2008) Intracellular mechanisms of specific beta-adrenoceptor antagonists involved in improved cardiac function and survival in a genetic model of heart failure. J Mol Cell Cardiol 45:240–249. doi:10.1016/j.yjmcc.2008.05.011
Belge C, Hammond J, Dubois-Deruy E, Manoury B, Hamelet J, Beauloye C, Markl A, Pouleur AC, Bertrand L, Esfahani H, Jnaoui K, Gotz KR, Nikolaev VO, Vanderper A, Herijgers P, Lobysheva I, Iaccarino G, Hilfiker-Kleiner D, Tavernier G, Langin D, Dessy C, Balligand JL (2013) Enhanced expression of beta3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation 129:451–462. doi:10.1161/CIRCULATIONAHA.113.004940
Bohm M, Deutsch HJ, Hartmann D, Rosee KL, Stablein A (1997) Improvement of postreceptor events by metoprolol treatment in patients with chronic heart failure. J Am Coll Cardiol 30:992–996
Bundgaard H, Liu CC, Garcia A, Hamilton EJ, Huang Y, Chia KK, Hunyor SN, Figtree GA, Rasmussen HH (2010) beta(3) adrenergic stimulation of the cardiac Na+-K+ pump by reversal of an inhibitory oxidative modification. Circulation 122:2699–2708. doi:10.1161/CIRCULATIONAHA.110.964619
Cheng HJ, Zhang ZS, Onishi K, Ukai T, Sane DC, Cheng CP (2001) Upregulation of functional beta(3)-adrenergic receptor in the failing canine myocardium. Circ Res 89:599–606
Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C (2004) Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 363:1365–1367. doi:10.1016/S0140-6736(04)16048-0
Datar R, Kaesemeyer WH, Chandra S, Fulton DJ, Caldwell RW (2010) Acute activation of eNOS by statins involves scavenger receptor-B1, G protein subunit Gi, phospholipase C and calcium influx. Br J Pharmacol 160:1765–1772. doi:10.1111/j.1476-5381.2010.00817.x
Feron O, Michel JB, Sase K, Michel T (1998) Dynamic regulation of endothelial nitric oxide synthase: complementary roles of dual acylation and caveolin interactions. Biochemistry 37:193–200. doi:10.1021/bi972307p
Feve B, Emorine LJ, Lasnier F, Blin N, Baude B, Nahmias C, Strosberg AD, Pairault J (1991) Atypical beta-adrenergic receptor in 3T3-F442A adipocytes. Pharmacological and molecular relationship with the human beta 3-adrenergic receptor. J Biol Chem 266:20329–20336
Fraccarollo D, Galuppo P, Schmidt I, Ertl G, Bauersachs J (2005) Additive amelioration of left ventricular remodeling and molecular alterations by combined aldosterone and angiotensin receptor blockade after myocardial infarction. Cardiovasc Res 67:97–105. doi:10.1016/j.cardiores.2005.03.001
Garcia-Prieto J, Garcia-Ruiz JM, Sanz-Rosa D, Pun A, Garcia-Alvarez A, Davidson SM, Fernandez-Friera L, Nuno-Ayala M, Fernandez-Jimenez R, Bernal JA, Izquierdo-Garcia JL, Jimenez-Borreguero J, Pizarro G, Ruiz-Cabello J, Macaya C, Fuster V, Yellon DM, Ibanez B (2014) beta3 adrenergic receptor selective stimulation during ischemia/reperfusion improves cardiac function in translational models through inhibition of mPTP opening in cardiomyocytes. Basic Res Cardiol 109:422. doi:10.1007/s00395-014-0422-0
Gauthier C, Leblais V, Kobzik L, Trochu JN, Khandoudi N, Bril A, Balligand JL, Le Marec H (1998) The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 102:1377–1384. doi:10.1172/JCI2191
George I, Sabbah HN, Xu K, Wang N, Wang J (2011) beta-adrenergic receptor blockade reduces endoplasmic reticulum stress and normalizes calcium handling in a coronary embolization model of heart failure in canines. Cardiovasc Res 91:447–455. doi:10.1093/cvr/cvr106
Hankes GH, Ardell JL, Tallaj J, Wei CC, Aban I, Holland M, Rynders P, Dillon R, Cardinal R, Hoover DB, Armour JA, Husain A, Dell’Italia LJ (2006) Beta1-adrenoceptor blockade mitigates excessive norepinephrine release into cardiac interstitium in mitral regurgitation in dog. Am J Physiol Heart Circ Physiol 291:H147–H151. doi:10.1152/ajpheart.00951.2005
Heilbrunn SM, Shah P, Bristow MR, Valantine HA, Ginsburg R, Fowler MB (1989) Increased beta-receptor density and improved hemodynamic response to catecholamine stimulation during long-term metoprolol therapy in heart failure from dilated cardiomyopathy. Circulation 79:483–490. doi:10.1161/01.CIR.79.3.483
Heusch G (2011) Beta3-adrenoceptor activation just says NO to myocardial reperfusion injury. J Am Coll Cardiol 58:2692–2694. doi:10.1016/j.jacc.2011.09.034
Hurt KJ, Sezen SF, Lagoda GF, Musicki B, Rameau GA, Snyder SH, Burnett AL (2012) Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci USA 109:16624–16629. doi:10.1073/Pnas.1213790109
Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ (1998) Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation 98:1783–1789. doi:10.1161/01.CIR.98.17.1783
Ishizaka N, Griendling KK, Lassegue B, Alexander RW (1998) Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation. Hypertension 32:459–466
Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, Groszmann RJ, Shah VH, Sessa WC (2006) Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci USA 103:19777–19782. doi:10.1073/pnas.0605907103
Jones AW, Durante W, Korthuis RJ (2010) Heme oxygenase-1 deficiency leads to alteration of soluble guanylate cyclase redox regulation. J Pharmacol Exp Ther 335:85–91. doi:10.1124/jpet.110.169755
Kleaveland JP, Kussmaul WG, Vinciguerra T, Diters R, Carabello BA (1988) Volume overload hypertrophy in a closed-chest model of mitral regurgitation. Am J Physiol 254:H1034–H1041
Kohout TA, Takaoka H, McDonald PH, Perry SJ, Mao L, Lefkowitz RJ, Rockman HA (2001) Augmentation of cardiac contractility mediated by the human beta(3)-adrenergic receptor overexpressed in the hearts of transgenic mice. Circulation 104:2485–2491
Koitabashi N, Arai M, Tomaru K, Takizawa T, Watanabe A, Niwano K, Yokoyama T, Wuytack F, Periasamy M, Nagai R, Kurabayashi M (2005) Carvedilol effectively blocks oxidative stress-mediated downregulation of sarcoplasmic reticulum Ca2+-ATPase 2 gene transcription through modification of Sp1 binding. Biochem Biophys Res Commun 328:116–124. doi:10.1016/j.bbrc.2004.12.139
Le DE, Pascotto M, Leong-Poi H, Sari I, Micari A, Kaul S (2013) Anti-inflammatory and pro-angiogenic effects of beta blockers in a canine model of chronic ischemic cardiomyopathy: comparison between carvedilol and metoprolol. Basic Res Cardiol 108:384. doi:10.1007/s00395-013-0384-7
Liu Y, Dillon AR, Tillson M, Makarewich C, Nguyen V, Dell’Italia L, Sabri AK, Rizzo V, Tsai EJ (2013) Volume overload induces differential spatiotemporal regulation of myocardial soluble guanylyl cyclase in eccentric hypertrophy and heart failure. J Mol Cell Cardiol 60:72–83. doi:10.1016/j.yjmcc.2013.03.019
Macdougall DA, Agarwal SR, Stopford EA, Chu H, Collins JA, Longster AL, Colyer J, Harvey RD, Calaghan S (2012) Caveolae compartmentalise beta2-adrenoceptor signals by curtailing cAMP production and maintaining phosphatase activity in the sarcoplasmic reticulum of the adult ventricular myocyte. J Mol Cell Cardiol 52:388–400. doi:10.1016/j.yjmcc.2011.06.014
Maczewski M, Mackiewicz U (2008) Effect of metoprolol and ivabradine on left ventricular remodelling and Ca2+ handling in the post-infarction rat heart. Cardiovasc Res 79:42–51. doi:10.1093/cvr/cvn057
Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, Rizzo V, Molkentin JD, Houser SR (2012) A caveolae-targeted L-type Ca(2)+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circ Res 110:669–674. doi:10.1161/CIRCRESAHA.111.264028
Markandeya YS, Fahey JM, Pluteanu F, Cribbs LL, Balijepalli RC (2011) Caveolin-3 regulates protein kinase A modulation of the Ca(V)3.2 (alpha1H) T-type Ca2+ channels. J Biol Chem 286:2433–2444. doi:10.1074/jbc.M110.182550
Mehta RH, Supiano MA, Oral H, Grossman PM, Montgomery DS, Smith MJ, Starling MR (2003) Compared with control subjects, the systemic sympathetic nervous system is activated in patients with mitral regurgitation. Am Heart J 145:1078–1085. doi:10.1016/S0002-8703(03)00111-X
Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL (2001) Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 103:1649–1655
Monto F, Oliver E, Vicente D, Rueda J, Aguero J, Almenar L, Ivorra MD, Barettino D, D’Ocon P (2012) Different expression of adrenoceptors and GRKs in the human myocardium depends on heart failure etiology and correlates to clinical variables. Am J Physiol Heart Circ Physiol 303:H368–H376. doi:10.1152/ajpheart.01061.2011
Muzzin P, Revelli JP, Fraser CM, Giacobino JP (1992) Radioligand binding studies of the atypical beta 3-adrenergic receptor in rat brown adipose tissue using [3H]CGP 12177. FEBS Lett 298:162–164. doi:10.1016/0014-5793(92)80046-J
Nagatomo Y, Yoshikawa T, Kohno T, Yoshizawa A, Anzai T, Meguro T, Satoh T, Ogawa S (2007) Effects of beta-blocker therapy on high sensitivity c-reactive protein, oxidative stress, and cardiac function in patients with congestive heart failure. J Card Fail 13:365–371. doi:10.1016/j.cardfail.2007.02.004
Nahmias C, Blin N, Elalouf JM, Mattei MG, Strosberg AD, Emorine LJ (1991) Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J 10:3721–3727
Napp A, Brixius K, Pott C, Ziskoven C, Boelck B, Mehlhorn U, Schwinger RH, Bloch W (2009) Effects of the beta3-adrenergic agonist BRL 37344 on endothelial nitric oxide synthase phosphorylation and force of contraction in human failing myocardium. J Card Fail 15:57–67. doi:10.1016/j.cardfail.2008.08.006
Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, Ellis CL, Miller KL, Vandegaer K, Bedja D, Gabrielson KL, Paolocci N, Kass DA, Barouch LA (2012) Cardioprotective effect of beta-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol 59:1979–1987. doi:10.1016/j.jacc.2011.12.046
Oh P, Schnitzer JE (1999) Immunoisolation of caveolae with high affinity antibody binding to the oligomeric caveolin cage. Toward understanding the basis of purification. J Biol Chem 274:23144–23154. doi:10.1074/jbc.274.33.23144
Pat B, Killingsworth C, Denney T, Zheng J, Powell P, Tillson M, Dillon AR, Dell’Italia LJ (2008) Dissociation between cardiomyocyte function and remodeling with beta-adrenergic receptor blockade in isolated canine mitral regurgitation. Am J Physiol Heart Circ Physiol 295:H2321–H2327. doi:10.1152/ajpheart.00746.2008
Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF (2003) Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol 285:H1720–H1729. doi:10.1152/ajpheart.00344.2002
Rybin VO, Xu X, Lisanti MP, Steinberg SF (2000) Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem 275:41447–41457
Sabri A, Rafiq K, Seqqat R, Kolpakov MA, Dillon R, Dell’italia LJ (2008) Sympathetic activation causes focal adhesion signaling alteration in early compensated volume overload attributable to isolated mitral regurgitation in the dog. Circ Res 102:1127–1136
Sato M, Hutchinson DS, Halls ML, Furness SG, Bengtsson T, Evans BA, Summers RJ (2012) Interaction with caveolin-1 modulates G protein coupling of mouse beta3-adrenoceptor. J Biol Chem 287:20674–20688. doi:10.1074/jbc.M111.280651
Schmidt K, Neubauer A, Kolesnik B, Stasch JP, Werner ER, Gorren AC, Mayer B (2012) Tetrahydrobiopterin protects soluble guanylate cyclase against oxidative inactivation. Mol Pharmacol 82:420–427. doi:10.1124/mol.112.079855
Schwencke C, Okumura S, Yamamoto M, Geng YJ, Ishikawa Y (1999) Colocalization of beta-adrenergic receptors and caveolin within the plasma membrane. J Cell Biochem 75:64–72
Sharma V, Dhillon P, Wambolt R, Parsons H, Brownsey R, Allard MF, McNeill JH (2008) Metoprolol improves cardiac function and modulates cardiac metabolism in the streptozotocin-diabetic rat. Am J Physiol Heart Circ Physiol 294:H1609–H1620. doi:10.1152/ajpheart.00949.2007
Sharma V, Parsons H, Allard MF, McNeill JH (2008) Metoprolol increases the expression of beta(3)-adrenoceptors in the diabetic heart: effects on nitric oxide signaling and forkhead transcription factor-3. Eur J Pharmacol 595:44–51. doi:10.1016/j.ejphar.2008.07.042
Sharma V, Sharma A, Saran V, Bernatchez PN, Allard MF, McNeill JH (2011) beta-receptor antagonist treatment prevents activation of cell death signaling in the diabetic heart independent of its metabolic actions. Eur J Pharmacol 657:117–125. doi:10.1016/j.ejphar.2011.01.044
Spoladore R, Fragasso G, Perseghin G, De Cobelli F, Esposito A, Maranta F, Calori G, Locatelli M, Lattuada G, Scifo P, Del Maschio A, Margonato A (2013) Beneficial effects of beta-blockers on left ventricular function and cellular energy reserve in patients with heart failure. Fundam Clin Pharmacol 27:455–464. doi:10.1111/j.1472-8206.2012.01029.x
Szajerski P, Zielonka J, Sikora A, Adamus J, Marcinek A, Gebicki J, Kozlovski VI, Drelicharz L, Chlopicki S (2006) Radical scavenging and NO-releasing properties of selected beta-adrenoreceptor antagonists. Free Radic Res 40:741–752
Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, Dostmann WR, Smith CJ, Kass DA (2005) cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res 96:100–109. doi:10.1161/01.RES.0000152262.22968.72
Thunemann M, Wen L, Hillenbrand M, Vachaviolos A, Feil S, Ott T, Han X, Fukumura D, Jain RK, Russwurm M, de Wit C, Feil R (2013) Transgenic mice for cGMP imaging. Circ Res 113:365–371. doi:10.1161/CIRCRESAHA.113.301063
Tsai EJ, Liu Y, Koitabashi N, Bedja D, Danner T, Jasmin JF, Lisanti MP, Friebe A, Takimoto E, Kass DA (2012) Pressure-overload-induced subcellular relocalization/oxidation of soluble guanylyl cyclase in the heart modulates enzyme stimulation. Circ Res 110:295–303
Vaniotis G, Glazkova I, Merlen C, Smith C, Villeneuve LR, Chatenet D, Therien M, Fournier A, Tadevosyan A, Trieu P, Nattel S, Hebert TE, Allen BG (2013) Regulation of cardiac nitric oxide signaling by nuclear beta-adrenergic and endothelin receptors. J Mol Cell Cardiol 62:58–68. doi:10.1016/j.yjmcc.2013.05.003
Waagstein F, Caidahl K, Wallentin I, Bergh CH, Hjalmarson A (1989) Long-term beta-blockade in dilated cardiomyopathy. Effects of short- and long-term metoprolol treatment followed by withdrawal and readministration of metoprolol. Circulation 80:551–563. doi:10.1161/01.CIR.80.3.551
Watts VL, Sepulveda FM, Cingolani OH, Ho AS, Niu X, Kim R, Miller KL, Vandegaer K, Bedja D, Gabrielson KL, Rameau G, O’Rourke B, Kass DA, Barouch LA (2013) Anti-hypertrophic and anti-oxidant effect of beta3-adrenergic stimulation in myocytes requires differential neuronal NOS phosphorylation. J Mol Cell Cardiol 62:8–17. doi:10.1016/j.yjmcc.2013.04.025
Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS (2007) Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell 129:511–522. doi:10.1016/j.cell.2007.02.046
Wu JL, Liu WZ, Liu JH, Qiao LY, Yuan YN (2011) Distribution and quantification of beta-3 adrenergic receptor in tissues of sheep. Animal 5:88–93. doi:10.1017/S1751731110001564
Yeh DC, Duncan JA, Yamashita S, Michel T (1999) Depalmitoylation of endothelial nitric-oxide synthase by acyl-protein thioesterase 1 is potentiated by Ca(2+)-calmodulin. J Biol Chem 274:33148–33154
Zhao Q, Wu TG, Jiang ZF, Chen GW, Lin Y, Wang LX (2007) Effect of beta-blockers on beta3-adrenoceptor expression in chronic heart failure. Cardiovasc Drugs Ther 21:85–90. doi:10.1007/s10557-007-6016-4
Zhao Q, Zeng F, Liu JB, He Y, Li B, Jiang ZF, Wu TG, Wang LX (2013) Upregulation of beta3-adrenergic receptor expression in the atrium of rats with chronic heart failure. J Cardiovasc Pharmacol Ther 18:133–137. doi:10.1177/1074248412460123
Zheng J, Yancey DM, Ahmed MI, Wei CC, Powell PC, Shanmugam M, Gupta H, Lloyd SG, McGiffin DC, Schiros CG, Denney TS Jr, Babu GJ, Dell’italia LJ (2014) Increased sarcolipin expression and adrenergic drive in humans with preserved left ventricular ejection fraction and chronic isolated mitral regurgitation. Circ Heart Fail 7:194–202. doi:10.1161/CIRCHEARTFAILURE.113.000519
Acknowledgments
We thank K. Joseph Hurt for providing the p-nNOS antibody; Johannes-Peter Stasch for providing BAY 60-2770; and Doug Tilley for reviewing the manuscript. This research was supported by the American Heart Association (Post-Doctoral Fellowship to D.M. Trappanese, Scientist Development Grant to E.J. Tsai), NIH (T32-HL091804 to S.R. Houser; R37-HL061690, R01-HL085503, P01-HL075443, and P01-HL108806 to W.J. Koch; P01-HL74237 and R01-HL108213 to F.A. Recchia; P50-HL077100 to L. Dell’Italia; K08-HL109159 to E.J. Tsai), and the Pennsylvania Department of Health (E.J. Tsai).
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. M. Trappanese and Y. Liu contributed equally.
Rights and permissions
About this article
Cite this article
Trappanese, D.M., Liu, Y., McCormick, R.C. et al. Chronic β1-adrenergic blockade enhances myocardial β3-adrenergic coupling with nitric oxide-cGMP signaling in a canine model of chronic volume overload: new insight into mechanisms of cardiac benefit with selective β1-blocker therapy. Basic Res Cardiol 110, 456 (2015). https://doi.org/10.1007/s00395-014-0456-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-014-0456-3